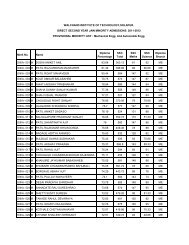
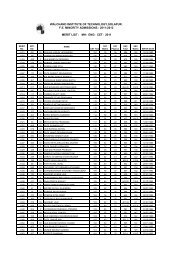
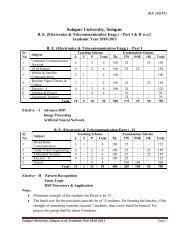
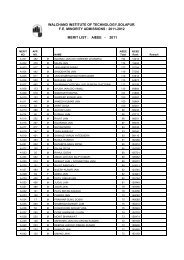
Model Question Paper CET Physics - WIT Solapur
Model Question Paper CET Physics - WIT Solapur
Model Question Paper CET Physics - WIT Solapur
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
33. The dry ice is :(1) Solid H 2 O (2) Solid CO 2 (3) Solid & Dry H 2 O (4) none of above→34. For the reaction 2A ← C + D the value of equilibrium constant is 1 x 10 -3 . If [C] = 1.2 x 10-3 M,[D] = 3.8 x 10 -6 M the value of [A] will be :(1) 5.2 x 10 -6 M (2) 3.6 x 10 -9 M(3) 2.1 x 10 -3 M (4) 4.8 x 10 -12 M35. Which of the following does not obey the octet rule :(1) PCI 3 (2) SF 6 (3) SO 2 (4) OF 236. Mustard gas is found from :(1) C 2 H 4 & H 2 SO 4 (2) C 2 H 4 & H 2 S(3) C 2 H 4 & S 2 C1 2 (4) C 2 H 4 & CH 3 SH37. The most reactive metal is :(1) Li (2) Au (3) F (4) Pt38. Which of the following has highest melting point :(1) C 4 H 10 (2) C 3 H 8 (3) C 2 H 6 (4) CH 439. Which of the following is not a metal :(1) Au (2) Hg (3) Ag (4) none of these40. In which of the following there is strong bond :(1) C=C (2) C-C (3) C≡C (4) all same41. The shape and size of 2p, 3p, 4p and 5p orbital are :(1) only equal in d block(2) equal in s block and different in p block(3) different(4) equal42. Malachite is a ore of :(1) Cu (2) Au (3) Ag (4) Mg43. If the ionization constant of CH 3 COOH is 1.8 x 10 5 , the degree of ionization of 0.01 MCH 3 COOh will be :(1) 1.8 x 10 -7 (2) 1.8 (3) 4.2 x 10 -2 (4) 42.4 x 10 -544. If the price of Nac1 sugar are 2 and 14 rupees per kg. then the price of 1 mole NaC1 and 1mole sugar will be :(1) 7 Rs. (2) different (3) equal (4) 28 Rs.45. In which of the following there are minimum nos. of molecule :(1) 2 gm. H 2 (2) 8 gm. O 2 (3) 16 gm. CO 2 (4) 4 gm. N 246. In which of the following central atom uses sp 2 hybrid orbitals :(1) SbH 3 (2) NH 3 (3) PH 3 (4) + CH 3