Hydrogen and its competitors, 2004
Hydrogen and its competitors, 2004
Hydrogen and its competitors, 2004
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
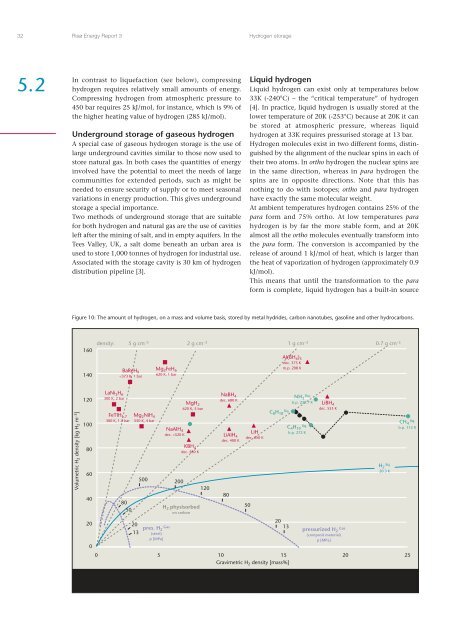
32Risø Energy Report 3<strong>Hydrogen</strong> storage5.2In contrast to liquefaction (see below), compressinghydrogen requires relatively small amounts of energy.Compressing hydrogen from atmospheric pressure to450 bar requires 25 kJ/mol, for instance, which is 9% ofthe higher heating value of hydrogen (285 kJ/mol).Underground storage of gaseous hydrogenA special case of gaseous hydrogen storage is the use oflarge underground cavities similar to those now used tostore natural gas. In both cases the quantities of energyinvolved have the potential to meet the needs of largecommunities for extended periods, such as might beneeded to ensure security of supply or to meet seasonalvariations in energy production. This gives undergroundstorage a special importance.Two methods of underground storage that are suitablefor both hydrogen <strong>and</strong> natural gas are the use of cavitiesleft after the mining of salt, <strong>and</strong> in empty aquifers. In theTees Valley, UK, a salt dome beneath an urban area isused to store 1,000 tonnes of hydrogen for industrial use.Associated with the storage cavity is 30 km of hydrogendistribution pipeline [3].Liquid hydrogenLiquid hydrogen can exist only at temperatures below33K (-240°C) – the “critical temperature” of hydrogen[4]. In practice, liquid hydrogen is usually stored at thelower temperature of 20K (-253°C) because at 20K it canbe stored at atmospheric pressure, whereas liquidhydrogen at 33K requires pressurised storage at 13 bar.<strong>Hydrogen</strong> molecules exist in two different forms, distinguishedby the alignment of the nuclear spins in each oftheir two atoms. In ortho hydrogen the nuclear spins arein the same direction, whereas in para hydrogen thespins are in opposite directions. Note that this hasnothing to do with isotopes; ortho <strong>and</strong> para hydrogenhave exactly the same molecular weight.At ambient temperatures hydrogen contains 25% of thepara form <strong>and</strong> 75% ortho. At low temperatures parahydrogen is by far the more stable form, <strong>and</strong> at 20Kalmost all the ortho molecules eventually transform intothe para form. The conversion is accompanied by therelease of around 1 kJ/mol of heat, which is larger thanthe heat of vaporization of hydrogen (approximately 0.9kJ/mol).This means that until the transformation to the paraform is complete, liquid hydrogen has a built-in sourceFigure 10: The amount of hydrogen, on a mass <strong>and</strong> volume basis, stored by metal hydrides, carbon nanotubes, gasoline <strong>and</strong> other hydrocarbons.160140BaReH 9520 K500 200MgH 2620 K, 5 barKBH 4dec. 580 KH 2 physisorbedon carbon120NaBH 4dec. 680 KLiAIH 4dec. 400 K80LiHdec. 650 K50C 8 H 18liq.NH liq. 3b.p. 239.7 KC 4 H liq. 10b.p. 272 KLiBH 4dec. 553 KH liq. 220.3 KCH 4liq.b.p. 112 K20002013pres. H Gas 2(steel)p [MPa]520131015Gravimetric H 2 density [mass%]pressurized H Gas 2(composit material)p [MPa]20