Chapter 5 Measurement of Atmospheric Pressure
Chapter 5 Measurement of Atmospheric Pressure
Chapter 5 Measurement of Atmospheric Pressure
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
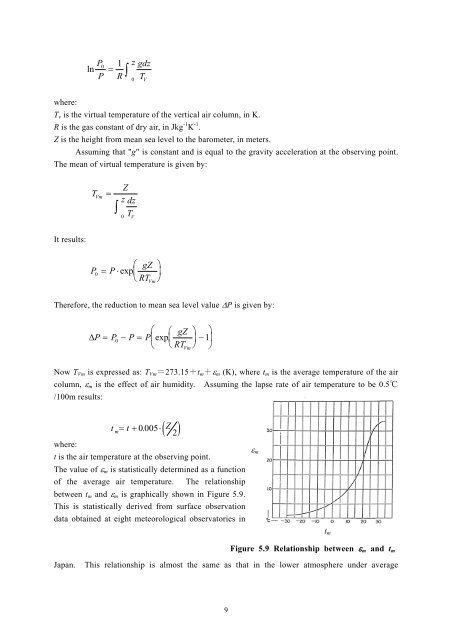
ln P 0P1Rz gdz0 T Vwhere:T v is the virtual temperature <strong>of</strong> the vertical air column, in K.R is the gas constant <strong>of</strong> dry air, in Jkg -1 K -1 .Z is the height from mean sea level to the barometer, in meters.Assuming that "g" is constant and is equal to the gravity acceleration at the observing point.The mean <strong>of</strong> virtual temperature is given by:TVmZz dzT0VIt results:P0 PexpgZRT VmTherefore, the reduction to mean sea level value P is given by: gZ P P0 P Pexp 1 RT VmNow T Vm is expressed as: T Vm =273.15+t m + m (K), where t m is the average temperature <strong>of</strong> the aircolumn, m is the effect <strong>of</strong> air humidity. Assuming the lapse rate <strong>of</strong> air temperature to be 0.5℃/100m results:tm t 0.005Z2where:t is the air temperature at the observing point.The value <strong>of</strong> m is statistically determined as a function<strong>of</strong> the average air temperature. The relationshipbetween t m and m is graphically shown in Figure 5.9.This is statistically derived from surface observationdata obtained at eight meteorological observatories in mt mFigure 5.9 Relationship between m and t mJapan. This relationship is almost the same as that in the lower atmosphere under average9




![関東地方の「紅葉の見ごろ予想」[PDF形式:250KB] - 気象庁](https://img.yumpu.com/47747063/1/184x260/pdf250kb-.jpg?quality=85)

![雨に関する各市町村の50年に一度の値一覧[PDF形式:417KB] - 気象庁](https://img.yumpu.com/47450161/1/190x134/50pdf417kb-.jpg?quality=85)
![1か月予報[九州北部地方]解説資料](https://img.yumpu.com/47153516/1/184x260/1.jpg?quality=85)
![職員募集案内パンフレット[PDF形式:約9MB] - 気象庁](https://img.yumpu.com/45714300/1/184x260/pdf9mb-.jpg?quality=85)


![ダウンロードファイル[PDF形式: 約15MB] - 気象庁](https://img.yumpu.com/43657928/1/190x135/pdf-15mb-.jpg?quality=85)