Factors affecting the complexation of polyacrylic ... - ResearchGate
Factors affecting the complexation of polyacrylic ... - ResearchGate
Factors affecting the complexation of polyacrylic ... - ResearchGate
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
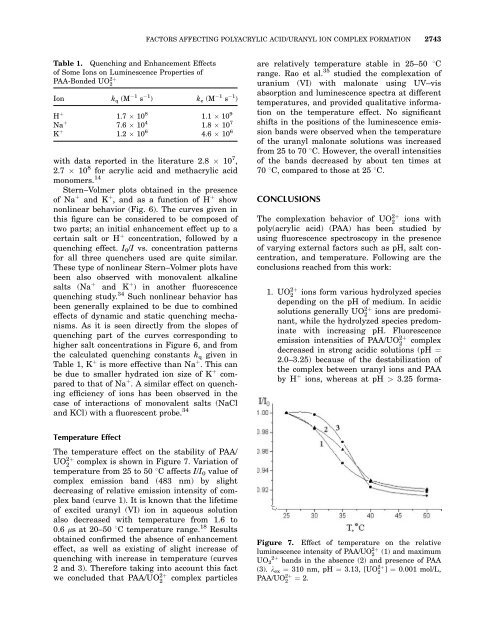
FACTORS AFFECTING POLYACRYLIC ACID/URANYL ION COMPLEX FORMATION 2743Table 1. Quenching and Enhancement Effects<strong>of</strong> Some Ions on Luminescence Properties <strong>of</strong>PAA-Bonded UO 2þ2Ion k q (M 1 s 1 ) k e (M 1 s 1 )H þ 1.7 10 8 1.1 10 9Na þ 7.6 10 4 1.8 10 7with data reported in <strong>the</strong> literature 2.8 10 7 ,2.7 10 8 for acrylic acid and methacrylic acidmonomers. 14Stern–Volmer plots obtained in <strong>the</strong> presence<strong>of</strong> Na þ and K þ , and as a function <strong>of</strong> H þ shownonlinear behavior (Fig. 6). The curves given inthis figure can be considered to be composed <strong>of</strong>two parts; an initial enhancement effect up to acertain salt or H þ concentration, followed by aquenching effect. I 0 /I vs. concentration patternsfor all three quenchers used are quite similar.These type <strong>of</strong> nonlinear Stern–Volmer plots havebeen also observed with monovalent alkalinesalts (Na þ and K þ ) in ano<strong>the</strong>r fluorescencequenching study. 34 Such nonlinear behavior hasbeen generally explained to be due to combinedeffects <strong>of</strong> dynamic and static quenching mechanisms.As it is seen directly from <strong>the</strong> slopes <strong>of</strong>quenching part <strong>of</strong> <strong>the</strong> curves corresponding tohigher salt concentrations in Figure 6, and from<strong>the</strong> calculated quenching constants k q given inTable 1, K þ is more effective than Na þ . This canbe due to smaller hydrated ion size <strong>of</strong> K þ comparedto that <strong>of</strong> Na þ . A similar effect on quenchingefficiency <strong>of</strong> ions has been observed in <strong>the</strong>case <strong>of</strong> interactions <strong>of</strong> monovalent salts (NaCland KCl) with a fluorescent probe. 34are relatively temperature stable in 25–50 8Crange. Rao et al. 35 studied <strong>the</strong> <strong>complexation</strong> <strong>of</strong>uranium (VI) with malonate using UV–visabsorption and luminescence spectra at differenttemperatures, and provided qualitative informationon <strong>the</strong> temperature effect. No significantshifts in <strong>the</strong> positions <strong>of</strong> <strong>the</strong> luminescence emissionbands were observed when <strong>the</strong> temperature<strong>of</strong> <strong>the</strong> uranyl malonate solutions was increasedfrom 25 to 70 8C. However, <strong>the</strong> overall intensities<strong>of</strong> <strong>the</strong> bands decreased by about ten times at70 8C, compared to those at 25 8C.CONCLUSIONSThe <strong>complexation</strong> behavior <strong>of</strong> UO 2þ2ions withpoly(acrylic acid) (PAA) has been studied byusing fluorescence spectroscopy in <strong>the</strong> presence<strong>of</strong> varying external factors such as pH, salt concentration,and temperature. Following are <strong>the</strong>conclusions reached from this work:1. UO 2þ2ions form various hydrolyzed speciesdepending on <strong>the</strong> pH <strong>of</strong> medium. In acidicsolutions generally UO 2þ2ions are predominant,while <strong>the</strong> hydrolyzed species predominatewith increasing pH. Fluorescenceemission intensities <strong>of</strong> PAA/UO 2þ2complexdecreased in strong acidic solutions (pH ¼2.0–3.25) because <strong>of</strong> <strong>the</strong> destabilization <strong>of</strong><strong>the</strong> complex between uranyl ions and PAAby H þ ions, whereas at pH > 3.25 forma-K þ 1.2 10 6 4.6 10 6 Figure 7. Effect <strong>of</strong> temperature on <strong>the</strong> relativeTemperature EffectThe temperature effect on <strong>the</strong> stability <strong>of</strong> PAA/UO 2þ2complex is shown in Figure 7. Variation <strong>of</strong>temperature from 25 to 50 8C affects I/I 0 value <strong>of</strong>complex emission band (483 nm) by slightdecreasing <strong>of</strong> relative emission intensity <strong>of</strong> complexband (curve 1). It is known that <strong>the</strong> lifetime<strong>of</strong> excited uranyl (VI) ion in aqueous solutionalso decreased with temperature from 1.6 to0.6 ls at 20–50 8C temperature range. 18 Resultsobtained confirmed <strong>the</strong> absence <strong>of</strong> enhancementeffect, as well as existing <strong>of</strong> slight increase <strong>of</strong>quenching with increase in temperature (curves2 and 3). Therefore taking into account this factwe concluded that PAA/UO 2þ2complex particlesluminescence intensity <strong>of</strong> PAA/UO 2þ2(1) and maximumUO 2þ 2 bands in <strong>the</strong> absence (2) and presence <strong>of</strong> PAA(3). k ex ¼ 310 nm, pH ¼ 3.13, [UO 2þ2] ¼ 0.001 mol/L,PAA/UO 2þ2¼ 2.