The isolation and characterization of organic c - Chemistry
The isolation and characterization of organic c - Chemistry
The isolation and characterization of organic c - Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
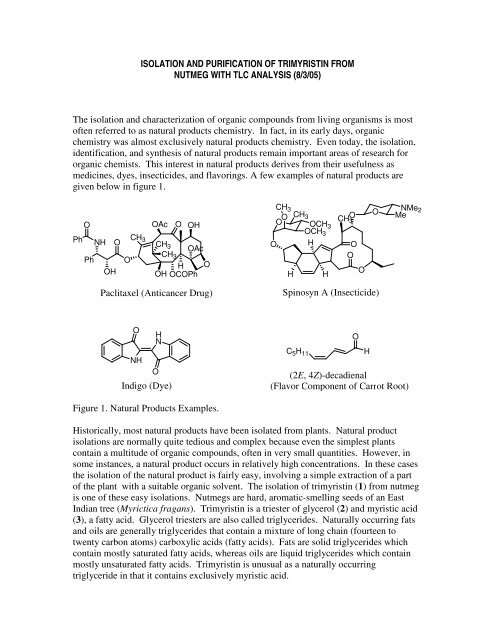
ISOLATION AND PURIFICATION OF TRIMYRISTIN FROMNUTMEG WITH TLC ANALYSIS (8/3/05)<strong>The</strong> <strong>isolation</strong> <strong>and</strong> <strong>characterization</strong> <strong>of</strong> <strong>organic</strong> compounds from living organisms is most<strong>of</strong>ten referred to as natural products chemistry. In fact, in its early days, <strong>organic</strong>chemistry was almost exclusively natural products chemistry. Even today, the <strong>isolation</strong>,identification, <strong>and</strong> synthesis <strong>of</strong> natural products remain important areas <strong>of</strong> research for<strong>organic</strong> chemists. This interest in natural products derives from their usefulness asmedicines, dyes, insecticides, <strong>and</strong> flavorings. A few examples <strong>of</strong> natural products aregiven below in figure 1.OPh NH OPhOHOAc O OHCH 3CH 3 OAcCHO3H OOH OCOPhCH 3OO O CH 3 CH 3 OCH3OCH 3O H OOHHOONMe 2MePaclitaxel (Anticancer Drug)Spinosyn A (Insecticide)ONHHNOIndigo (Dye)C 5 H 11(2E, 4Z)-decadienal(Flavor Component <strong>of</strong> Carrot Root)OHFigure 1. Natural Products Examples.Historically, most natural products have been isolated from plants. Natural product<strong>isolation</strong>s are normally quite tedious <strong>and</strong> complex because even the simplest plantscontain a multitude <strong>of</strong> <strong>organic</strong> compounds, <strong>of</strong>ten in very small quantities. However, insome instances, a natural product occurs in relatively high concentrations. In these casesthe <strong>isolation</strong> <strong>of</strong> the natural product is fairly easy, involving a simple extraction <strong>of</strong> a part<strong>of</strong> the plant with a suitable <strong>organic</strong> solvent. <strong>The</strong> <strong>isolation</strong> <strong>of</strong> trimyristin (1) from nutmegis one <strong>of</strong> these easy <strong>isolation</strong>s. Nutmegs are hard, aromatic-smelling seeds <strong>of</strong> an EastIndian tree (Myrictica fragans). Trimyristin is a triester <strong>of</strong> glycerol (2) <strong>and</strong> myristic acid(3), a fatty acid. Glycerol triesters are also called triglycerides. Naturally occurring fats<strong>and</strong> oils are generally triglycerides that contain a mixture <strong>of</strong> long chain (fourteen totwenty carbon atoms) carboxylic acids (fatty acids). Fats are solid triglycerides whichcontain mostly saturated fatty acids, whereas oils are liquid triglycerides which containmostly unsaturated fatty acids. Trimyristin is unusual as a naturally occurringtriglyceride in that it contains exclusively myristic acid.
OCH 2 OC(CH 2 ) 12 CH 3CH 2 OHOOCHOC(CH 2 ) 12 CH 3CHOHHOC (CH 2 ) 12 CH 3OCH 2 OC(CH 2 ) 12 CH 3CH 2 OH12 3Trimyristin Glycerol Myristic acidIn this experiment, a whole nutmeg will be crushed <strong>and</strong> extracted with diethyl ether toobtain crude trimyristin. <strong>The</strong> crude trimyristin will be purified by crystallization, <strong>and</strong> itspurity will be checked its melting point <strong>and</strong> by thin layer chromatography. Whentrimyristin is hydrolyzed with a base, a process known as saponification, glycerol <strong>and</strong>myristic acid are produced. Myristic acid has a melting point very close to that <strong>of</strong>trimyristin so that a melting point alone is not sufficient for distinguishing trimyristinfrom myristic acid. However trimyristin <strong>and</strong> myristic acid may be readily distinguishedby thin layer chromatography.Experimental ProcedureFirst LabCrush <strong>and</strong> grind a whole nutmeg with a mortar <strong>and</strong> pestle for approximately 5 min. (noneed to pulverize). Weigh the ground nutmeg <strong>and</strong> transfer it to a 50 mL round-bottomedflask. Add 15 mL <strong>of</strong> diethyl ether, attach a water-cooled condenser to the flask, <strong>and</strong> heatthe mixture under a gentle reflux for approximately 15 min. on a steam plate. Allow themixture to cool to room temperature. Without disturbing the settled solids, decant <strong>and</strong>gravity-filter the ether solution into a 125 mL Erlenmeyer flask. Add another 10 mL <strong>of</strong>diethyl ether to the solid residue, swirl for a few minutes, <strong>and</strong> transfer the contents to thefilter. Prepare a TLC plate <strong>and</strong> apply a small amount <strong>of</strong> the filtrate (crude product).(Wait until the following week to develop in a chamber.) Finally, add a boiling stick tothe filtrate <strong>and</strong> evaporate the ether on a steam plate in the fume hood.
Second LabAdd sufficient reagent grade acetone to dissolve the remaining crude solid product(approximately 3-5 mL <strong>of</strong> acetone, the amount will vary depending upon the weight <strong>of</strong>nutmeg extracted). Warm the mixture <strong>and</strong> allow to cool to room temperature. Collect thecrystalline trimyristin by vacuum filtration <strong>and</strong> allow it to air-dry. Weigh the product <strong>and</strong>calculate the percent <strong>of</strong> trimyristin in the nutmeg.Place a small amount (the tip <strong>of</strong> a spatula) <strong>of</strong> the purified trimyristin in a test tube, thenadd a drop or two <strong>of</strong> chlor<strong>of</strong>orm. Next, obtain a small amount <strong>of</strong> prepared myristic acid(saponified trimyristin) <strong>and</strong> dissolve in chlor<strong>of</strong>orm (same amounts/procedure as used fortrimyristin & crude product). Spot the TLC plate with each <strong>of</strong> the chlor<strong>of</strong>orm solutions<strong>and</strong> develop the plate using chlor<strong>of</strong>orm as the solvent. Place the plate under short UV light<strong>and</strong> observe. Next, develop the plate in an iodine chamber. Note that myristic acid adsorbsless iodine than the surrounding silica gel <strong>and</strong> appears as a white spot on a yellow-brownbackground.Place some dry trimyristin, just enough to see, in a melting point capillary. Do likewisewith a small amount <strong>of</strong> prepared myristic acid <strong>and</strong> determine the melting point <strong>of</strong> the twocompounds. <strong>The</strong> melting point should be near 56 o C. Remember that melting pointdeterminations are most accurate when the temperature rise near the melting point (within10 °C) is approximately 1-2 o C/min. Prepare the TLC plate in a manner consistant with those prepared in the lab’sintroductory experiment, “TLC <strong>of</strong> Analgesics”. Do not discard TLC plates. Attach to the postlab exercise form. Perform purification, TLC <strong>and</strong> melting point analysis during 2 nd week <strong>of</strong> thisexperiment. Always use a flask (not a beaker) to contain volatile reagents. A flask must always beused for crystallization/recrystallization procedures!WASTE DISPOSAL NOTES: Nutmeg, Trimyristin, <strong>and</strong> Myristic Acid should all be disposed <strong>of</strong> inthe Non-hazardous Solid Waste Container (located in the Waste Hood)
Figure 2: Reflux Apparatus (NOTE: Use a hose clamp to anchor hoses to the condenser inlet<strong>and</strong> outlets)
Due in the next lab. No exceptions will be made. Please answer questions on thisform. (30 pts TOTAL)
B. SUMMARY QUESTIONS (15pts)1) (4pts) Is melting point determination or thin-layer chromatography analysis abetter method to determine the purity <strong>of</strong> trimyristin? Explain.2) (4pts) Was the melting point or thin-layer chromatography a better method fordistinguishing between the compounds trimyristin <strong>and</strong> myristic acid? Explain.3) (4pts) Why was the whole nutmeg crushed <strong>and</strong> ground before it was extractedwith diethyl ether?4) (3pts) Would water be a suitable solvent for extracting trimyristin from nutmeg?