Bavarian Biotech Report 2011/2012 (7MB) - Bio M
Bavarian Biotech Report 2011/2012 (7MB) - Bio M
Bavarian Biotech Report 2011/2012 (7MB) - Bio M
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
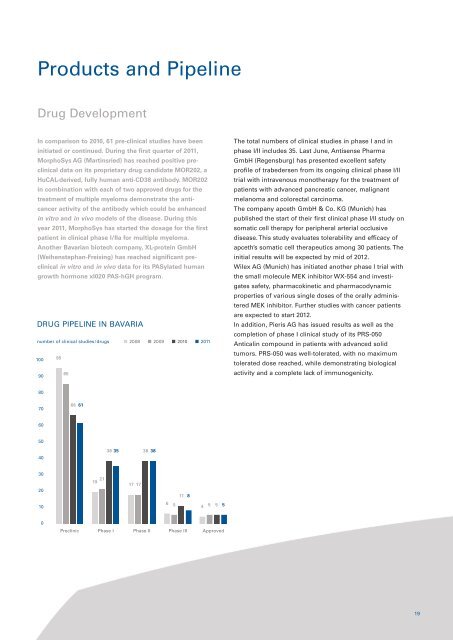
Products and PipelineDrug DevelopmentIn comparison to 2010, 61 pre-clinical studies have beeninitiated or continued. During the first quarter of <strong>2011</strong>,MorphoSys AG (Martinsried) has reached positive preclinicaldata on its proprietary drug candidate MOR202, aHuCAL-derived, fully human anti-CD38 antibody. MOR202in combination with each of two approved drugs for thetreatment of multiple myeloma demonstrate the anticanceractivity of the antibody which could be enhancedin vitro and in vivo models of the disease. During thisyear <strong>2011</strong>, MorphoSys has started the dosage for the firstpatient in clinical phase I/IIa for multiple myeloma.Another <strong>Bavarian</strong> biotech company, XL-protein GmbH(Weihenstephan-Freising) has reached significant preclinicalin vitro and in vivo data for its PASylated humangrowth hormone xI020 PAS-hGH program.DRUG PIPELINE IN BAVARIAnumber of clinical studies/drugs 2008 2009 2010 <strong>2011</strong>100 959085The total numbers of clinical studies in phase I and inphase I/II includes 35. Last June, Antisense PharmaGmbH (Regensburg) has presented excellent safetyprofile of trabedersen from its ongoing clinical phase I/IItrial with intravenous monotherapy for the treatment ofpatients with advanced pancreatic cancer, malignantmelanoma and colorectal carcinoma.The company apceth GmbH & Co. KG (Munich) haspublished the start of their first clinical phase I/II study onsomatic cell therapy for peripheral arterial occlusivedisease. This study evaluates tolerability and efficacy ofapceth’s somatic cell therapeutics among 30 patients. Theinitial results will be expected by mid of <strong>2012</strong>.Wilex AG (Munich) has initiated another phase I trial withthe small molecule MEK inhibitor WX-554 and investigatessafety, pharmacokinetic and pharmacodynamicproperties of various single doses of the orally administeredMEK inhibitor. Further studies with cancer patientsare expected to start <strong>2012</strong>.In addition, Pieris AG has issued results as well as thecompletion of phase I clinical study of its PRS-050Anticalin compound in patients with advanced solidtumors. PRS-050 was well-tolerated, with no maximumtolerated dose reached, while demonstrating biologicalactivity and a complete lack of immunogenicity.807066 6160504038 35 38 383020211917 17118106 54 5 5 50Preclinic Phase I Phase II Phase III Approved19