You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
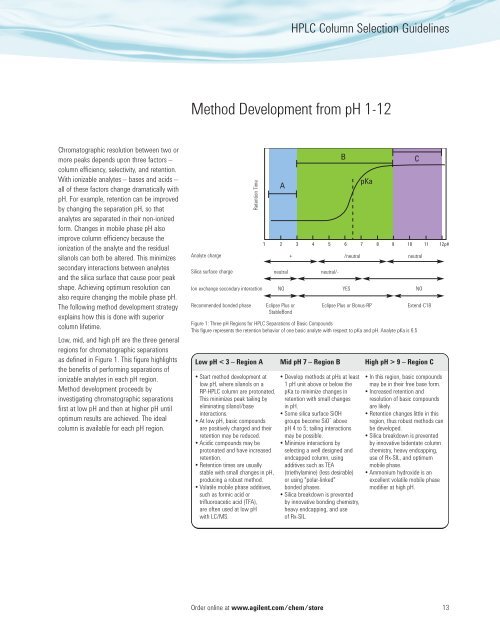
<strong>HPLC</strong> <strong>Column</strong> <strong>Selection</strong> <strong>Guide</strong>linesMethod Development from pH 1-12Chromatographic resolution between two ormore peaks depends upon three factors –column efficiency, selectivity, and retention.With ionizable analytes – bases and acids –all of these factors change dramatically withpH. For example, retention can be improvedby changing the separation pH, so thatanalytes are separated in their non-ionizedform. Changes in mobile phase pH alsoimprove column efficiency because theionization of the analyte and the residualsilanols can both be altered. This minimizessecondary interactions between analytesand the silica surface that cause poor peakshape. Achieving optimum resolution canalso require changing the mobile phase pH.The following method development strategyexplains how this is done with superiorcolumn lifetime.Low, mid, and high pH are the three generalregions for chromatographic separationsas defined in Figure 1. This figure highlightsthe benefits of performing separations ofionizable analytes in each pH region.Method development proceeds byinvestigating chromatographic separationsfirst at low pH and then at higher pH untiloptimum results are achieved. The idealcolumn is available for each pH region.Analyte chargeSilica surface chargeRetention TimeIon exchange secondary interactionRecommended bonded phaseneutralNOEclipse Plus orStableBond+ /neutral neutralneutral/-YESEclipse Plus or Bonus-RPFigure 1: Three pH Regions for <strong>HPLC</strong> Separations of Basic CompoundsThis figure represents the retention behavior of one basic analyte with respect to pKa and pH. Analyte pKa is 6.5NOExtend-C18Low pH < 3 – Region A Mid pH 7 – Region B High pH > 9 – Region C Start method development atlow pH, where silanols on aRP-<strong>HPLC</strong> column are protonated.This minimizes peak tailing byeliminating silanol/baseinteractions. At low pH, basic compoundsare positively charged and theirretention may be reduced. Acidic compounds may beprotonated and have increasedretention. Retention times are usuallystable with small changes in pH,producing a robust method. Volatile mobile phase additives,such as formic acid ortrifluoroacetic acid (TFA),are often used at low pHwith LC/MS. Develop methods at pHs at least1 pH unit above or below thepKa to minimize changes inretention with small changesin pH. Some silica surface SiOHgroups become SiO¯ abovepH 4 to 5; tailing interactionsmay be possible. Minimize interactions byselecting a well designed andendcapped column, usingadditives such as TEA(triethylamine) (less desirable)or using "polar-linked"bonded phases. Silica breakdown is preventedby innovative bonding chemistry,heavy endcapping, and useof Rx-SIL. In this region, basic compoundsmay be in their free base form. Increased retention andresolution of basic compoundsare likely. Retention changes little in thisregion, thus robust methods canbe developed. Silica breakdown is preventedby innovative bidentate columnchemistry, heavy endcapping,use of Rx-SIL, and optimummobile phase. Ammonium hydroxide is anexcellent volatile mobile phasemodifier at high pH.Order online at www.agilent.com/chem/store13