Lecture 3_s - TeachLine
Lecture 3_s - TeachLine
Lecture 3_s - TeachLine
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3OsmosisLowering [water]depends on thenumber of moleculesadded55.5Mglucose1mol glucose in 1Lwater reduces the[water] by 1M(54.5M)54.5MAdi Mizrahi, The Hebrew University of Jerusalem
4OsmosisLowering [water]depends on thenumber of moleculesadded1mol NaCl in 1L waterreduces the [water] by1mol Na + 1 mol Cl =2 Osmol(53.5M) 5M)55.5MNaCl53.5MAdi Mizrahi, The Hebrew University of Jerusalem
5Osmolarity1 M glucose = 1Osmol glucose1 M NaCl = 2 Osmol NaClOsmoticcoefficientוליתר דיוקOsmolarity = * i * CNumber of ions formedby dissociation of themoleculeMolarconcentrationtiof the soluteAdi Mizrahi, The Hebrew University of Jerusalem
6OsmolarityWhat is the osmolarity of 205mM NaCl solution ?Osmolarity = 0.93 x 2 x 205mM = 381.33 mOsmol * i * CAdi Mizrahi, The Hebrew University of Jerusalem
7Osmolarity205mM NaCl0.93 x 2 x 205mM = 381.3 mOsmol147.8mM CaCl 20.86 x 3 x 147.8mM = 381.33 mOsmolThe water concentration of 2 solution with the same osmolarity is equal !!!Adi Mizrahi, The Hebrew University of Jerusalem
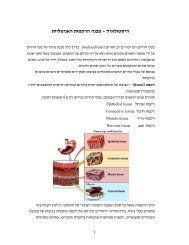
8Osmosis – the net diffusion of water acrosspermeable membranesInitialEquilibriumAdi Mizrahi, The Hebrew University of Jerusalem
10Osmotic Pressure is…..the pressure that is just enough to keep water fromflowing into the compartment with the lower waterconcentration (or higher solute concentration)Adi Mizrahi, The Hebrew University of Jerusalem
11Osmotic Pressurevan Hoff’s lawIdeal gasconstantOsmolarity = R x T xOsmoticpressureAbsoluteTemp.( ic)Adi Mizrahi, The Hebrew University of Jerusalem
12OsmoticityPhysiologically~300 mOsmol > 2 1 = 3 1
13Changes in cell volumes due to osmosis(tonicity)1 intracellularSemi-permeablemembrane(only to water) =212 Extra cellularAdi Mizrahi, The Hebrew University of Jerusalem
14Changes in cell volumes due to osmosis(tonicity)Will the cell expand ? 1Semi-permeablemembrane(only to water) =212Adi Mizrahi, The Hebrew University of Jerusalem
15Changes in cell volumes due to osmosis(tonicity)Will the cell expand ?1Semi-permeablemembrane(only to water) >212Adi Mizrahi, The Hebrew University of Jerusalemequilibrium
16Changes in cell volumes due to osmosis(tonicity)Will the cell expand ? 1Semi-permeablemembrane(only to water)
17Determining isotonicity to a red blood cellThe red blood cell isimpermeable to NaCl.What is the isotonicconcentration of NaClfor the RBC ?In the experiment, therelative change in cellvolume (V) is plottedagainst a gradient of[NaCl].V o - normal volume inplasmaAdi Mizrahi, The Hebrew University of JerusalemIsotonic NaCl concentration is 154mM
17Isotonic saline is 154 mMAdi Mizrahi, The Hebrew University of JerusalemIsotonic NaCl concentration is 154mM
19But are tonicity andosmoticity synonyms ?No !Adi Mizrahi, The Hebrew University of Jerusalem
20For example :Hyper-osmoticBUTIsotonicAdi Mizrahi, The Hebrew University of Jerusalem
21Non-Diffusion transport across membranesPinocytosis (cell “drinking”)Phagocytosis (cell “eating”)Adi Mizrahi, The Hebrew University of Jerusalem
22Transport in EpitheliaAdi Mizrahi, The Hebrew University of Jerusalem
23Transport in EpitheliaAdi Mizrahi, The Hebrew University of Jerusalem
24Transport in EpitheliaAdi Mizrahi, The Hebrew University of Jerusalem
25- מושגיםמס 3שעורמושגםOsmosis-Osmolarity-Osmostic pressure-Osmotic solutions (iso, hyper, hypo)-Tonicity (iso, hyper, hypo).Transport (2)-Exocytosis.-Endocytosis.-Transport via epitheliumAdi Mizrahi, The Hebrew University of Jerusalem