tolsurf-protocol - Associates in Newborn Medicine
tolsurf-protocol - Associates in Newborn Medicine
tolsurf-protocol - Associates in Newborn Medicine
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
TABLE OF CONTENTSSITE INVESTIGATORS 5GLOSSARY 6OVERVIEW 61.0 SCIENTIFIC BACKGROUND 71.1 Pathophysiology of Bronchopulmonary Dysplasia (BPD) 71.2 Epidemiology of BPD 81.3 Pulmonary surfactant 81.4 Pilot Trials 81.5 Inhaled Nitric Oxide for the prevention of BPD 101.5.a. Animal Studies 101.5.b. Cl<strong>in</strong>ical Trials 101.5.c. Prevention of BPD with the use of <strong>in</strong>haled Nitric Oxide (iNO):the NO CLD Trial1.5.d. NIH Consensus Conference on INO <strong>in</strong> the Premature Infant 101.6 Interaction Between Surfactant And Nitric Oxide 112.0 HYPOTHESIS AND SPECIFIC AIMS 122.1 Hypothesis and Rationale for Trial 122.2 Specific Aims 133.0 RESEARCH DESIGN 143.1 Sites Involved (see Appendix A p.35) 143.2 Site Activities 14Table 3.1 Study Procedure Chart 144.0 ELIGIBILITY 154.1 Inclusion Criteria 154.2 Exclusion Criteria 15Table 4.3 Def<strong>in</strong>ition of Cl<strong>in</strong>ically Unstable for TOLSURF 155.0 RECRUITMENT AND RANDOMIZATION 155.1 Recruitment 155.2 Accrual - Meet<strong>in</strong>g Recruitment Targets 155.3 Randomization 166.0 STUDY TREATMENT 176.1 Inhaled Nitric Oxide 176.2 Late Surfactant Treatment 176.3 Instillation Procedure 176.4 Bl<strong>in</strong>d<strong>in</strong>g of Surfactant/Placebo Adm<strong>in</strong>istration 176.5 Retreatment with Surfactant/Placebo 17PAGE1/18/2011 2
6.6 Patients Extubated with<strong>in</strong> 24 Days of Study Initiation 187.0 STUDY TREATMENT MODIFICATIONS 187.1 Indications for Interruption of Study Drug Adm<strong>in</strong>istration 187.2 Stopp<strong>in</strong>g Study Drug Adm<strong>in</strong>istration for Individual Patient 187.3 Open Label Use of Late Surfactant 188.0 OUTCOME MEASURES 188.1 Primary Outcome 188.2 Secondary Outcomes 198.2.a. Discharge home or off respiratory support 198.2.b. Severity of BPD 198.2.c. Pulmonary outcome at 12 and 20 m of age 198.2.d. Neurodevelopmental outcome at 22-26 mo of age 199.0 DATA COLLECTION AND EVALUATION 209.1 Cl<strong>in</strong>ical Data Collection 209.2 Other Cl<strong>in</strong>ical Management 209.3 Data Quality Assurance 2010.0 SAMPLES AND LABORATORY STUDIES 2110.1 Tracheal Aspirate Samples 2110.2 Ur<strong>in</strong>e Samples 2111.0 ADVERSE EVENT AND PROTOCOL VIOLATION REPORTING 2111.1 Adverse Event Report<strong>in</strong>g 2111.1.a. Serious Adverse Events (SAE) 2111.1.b. Adverse Events 21Table 11.1 Proposed SAE and AE Report<strong>in</strong>g 22Table 11.2 AE REPORTING – IN MOP 22Table 11.3 Serious Adverse Event/Adverse Event Flowchart 2311.2 Protocol Violation/Deviation Report<strong>in</strong>g 2411.2.a Major Protocol Violations 2411.2.b Protocol Deviations 2412.0 STATISTICAL CONSIDERATIONS 2512.1 Sample Size and Power 2512.1.a. Assumptions for calculation of sample size 2512.1.b. Placebo/ iNO-only event rate 2512.1.c. Treatment effect size 2512.1.d. Sample size results 2512.1.e Power and sample size under alternative <strong>in</strong>cidence 25and effect size assumptions12.2.General Statistical analysis considerations 2512.2.a. Cluster<strong>in</strong>g of outcomes with<strong>in</strong> multiple births 2512.2.b. Miss<strong>in</strong>g data 2612.2.c. Analyses restricted to surviv<strong>in</strong>g <strong>in</strong>fants rema<strong>in</strong><strong>in</strong>g <strong>in</strong> follow-up 2612.3 Statistical Analysis Plan: Primary Outcome 261/18/2011 3
12.4 Statistical Analysis Plan: Secondary Analyses of Primary Outcome 2612.4.a. Check<strong>in</strong>g balance by treatment on basel<strong>in</strong>e covariates 2612.4.b. Subgroup analyses 2712.5 Statistical Analysis Plan: Secondary Endpo<strong>in</strong>ts and Specific Aim 2712.5 a. 40 wk outcome- discharged home or off respiratory support 2712.5.b. Severity of BPD 2712.5.c. Pulmonary status at 12 and 20 months of age 2712.5.d. Neurodevelopmental impairment (NDI) at 22 - 26 months of age 2712.5.e. Changes <strong>in</strong> surfactant levels (Specific Aim 2) 2712.6 Exploratory Analyses 2713.0 DATA COLLECTION AND MANAGEMENT 2813.1 Data Collection 2813.2 Data Management 2813.3 Data Audit 2813.4 Data Security And Confidentiality 2813.4.a. Consent 2913.4.b. Subject identifiers 2913.4.c. Data submission 2914.0 DOCUMENTATION AND RECORD RETENTION 3014.1 Documentation 3014.2 Record Retention 3015.0 INTERIM SUMMARIES AND ANALYSES 3015.1 Interim Summaries 3015.2 Interim Analysis 3016.0 PROTOCOL AMENDMENTS 3116.1 Other Changes In Study Conduct 3117.0 STUDY ORGANIZATION AND ADMINISTRATION 3118.0 POTENTIAL RISKS, BENEFITS AND ALTERNATIVES 3118.1 Potential Risks 3118.2 Potential Benefits 3118.3 Alternatives 3219.0 FINANCIAL CONSIDERATIONS 3220.0 DRUG HANDLING AND STORAGE 3221.0 FEDERAL OVERSIGHT 3222.0 REFERENCES 321/18/2011 4
Site InvestigatorsRoberta Keller, M.D.*Children’s HospitalUniversity of California San FranciscoSan Francisco, CAWilliam Truog M.D.*Children’s Mercy HospitalKansas City, MORob<strong>in</strong> Ste<strong>in</strong>horn, M.D.*Children’s Memorial and Northwestern University HospitalsChicago, ILEric Eichenwald, M.D.*Jeffrey Merrill*/David Durand, M.D.*Children’s Hospital and Research Center, Oakland CAAlta Bates Summit Medical Center, Berkeley CAAnn Marie Reynolds M.D.State University of New York at Buffalo andWoman and Children’s Hospital ofBuffalo, NYSherry Courtney, M.D.Stony Brook University Medical CenterStony Brook, NYDennis Mayock/Chris GleasonUniversity of Wash<strong>in</strong>gtonSeattle, WAMark HudakWolfson Children’s Hospital and Shands HospitalJacksonvillle, FLMark Mammel*/Andrea LamplandChildren’s Hospital and Cl<strong>in</strong>ics of M<strong>in</strong>nesotaSt Paul, MNEllen Bendel-Stenzel/ Neil MulrooneyChildren’s Hospital and Cl<strong>in</strong>icsM<strong>in</strong>neapolis, MNCarol Wagner/ Francis Row<strong>in</strong>sky KochMedical University of South Carol<strong>in</strong>aCharleston, SCMike O’Shea**/Beatrice StefanescuWake Forest UniversityW<strong>in</strong>ston Salem, NCReddy DhanireddyUT MemphisMemphis, TN1/18/2011 5
Cathy Bendel/Mike Georgieff**University of M<strong>in</strong>nesotaM<strong>in</strong>neapolis, MN* = member of steer<strong>in</strong>g committee, ** = followup steer<strong>in</strong>gGLOSSARYBPD bronchopulmonary dysplasia NNT number needed to treatCCC cl<strong>in</strong>ical coord<strong>in</strong>at<strong>in</strong>g center NO nitric oxideCI confidence <strong>in</strong>terval OI oxygenation <strong>in</strong>dex [(MAPxFIO 2 )/PaO 2 ]CRF case report form OR odds ratioDCC data coord<strong>in</strong>at<strong>in</strong>g center PDA patent ductus arteriosusDPPC Dipalmitoylphosphatidylchol<strong>in</strong>e PL phospholipidELGAN Extremely low gestational age newborn PMA post menstrual ageFIO2 fraction of <strong>in</strong>spired oxygen PVL periventricular leukomalaciaiNO <strong>in</strong>haled nitric oxide RDS respiratory distress syndromeIVH <strong>in</strong>traventricular hemorhage ROP ret<strong>in</strong>opathy of prematurityMAP mean airway pressure RSS respiratory severity score (MAPxFIO 2 )mRNA messenger ribonucleic acid SP surfactant prote<strong>in</strong>NCPAP nasal cont<strong>in</strong>uous positive airway pressure STads adsorption surface tensionNEC necrotiz<strong>in</strong>g enterocolitis STmax maximum surface tensionNICU neonatal <strong>in</strong>tensive care unit STm<strong>in</strong> M<strong>in</strong>imum surface tensionOVERVIEWInfants born prematurely are at risk for both respiratory distress syndrome (RDS) andbronchopulmonary dysplasia (BPD), def<strong>in</strong>ed as a cont<strong>in</strong>u<strong>in</strong>g requirement for ventilatory supportand/or supplemental oxygen at 36 wk postmenstrual age (PMA). BPD affects more than 70% of<strong>in</strong>fants of < 28 wk gestation who require ventilatory support after 7 d of age. Severe forms of BPD areassociated with long-term pulmonary disability, neurodevelopmental abnormalities and death. It isestimated that there are up to 15,000 new cases of BPD annually <strong>in</strong> the United States.Most very premature newborn <strong>in</strong>fants are deficient <strong>in</strong> pulmonary surfactant at birth, and currentcl<strong>in</strong>ical care <strong>in</strong>cludes surfactant replacement therapy to reduce the <strong>in</strong>cidence and severity of RDS.Despite surfactant treatment at birth, premature <strong>in</strong>fants often need mechanical ventilatory supportand/or supplemental oxygen dur<strong>in</strong>g the first wk of life and many have a cont<strong>in</strong>u<strong>in</strong>g requirement forrespiratory support after the first wk, often requir<strong>in</strong>g re<strong>in</strong>tubation or <strong>in</strong>creased ventilatory support. Wefound that most of these <strong>in</strong>fants experienced respiratory deteriorations that were associated withdysfunctional surfactant and low content of surfactant prote<strong>in</strong>s (SP) B and C 1 . Although <strong>in</strong>haled nitricoxide (iNO) started between 7 and 14 d of age significantly improves outcome <strong>in</strong> this group of <strong>in</strong>fants2,3 , these <strong>in</strong>fants still have episodes of surfactant dysfunction.We propose that episodes of surfactant dysfunction <strong>in</strong> chronically ventilated <strong>in</strong>fants contribute todevelopment of BPD by <strong>in</strong>creas<strong>in</strong>g lung <strong>in</strong>flammation and <strong>in</strong>jury secondary to greater exposure tooxygen and volutrauma, and by restrict<strong>in</strong>g distribution of iNO secondary to atelectasis. Based onthese observations, we propose to conduct a multicenter, randomized, controlled trial of surfactanttreatment for 524 <strong>in</strong>fants
Specific Aim 1 will assess the effect of late doses of surfactant <strong>in</strong> <strong>in</strong>fants receiv<strong>in</strong>g <strong>in</strong>haled iNO onsurvival without BPD <strong>in</strong> ventilated extremely low gestational age newborn (ELGANS) <strong>in</strong>fants.Aim 2 assess effects of late surfactant treatment on surfactant status and lung <strong>in</strong>flammatorybiomarkers and establishes a DNA repository for genomic studies of the pathogenesis of BPD.1.0 SCIENTIFIC BACKGROUND1.1 Pathophysiology of Bronchopulmonary Dysplasia (BPD). Infants born prematurely often haverespiratory failure because of structurally immature lungs, deficiency of pulmonary surfactant, primitiverespiratory drive, disturbed <strong>in</strong>trauter<strong>in</strong>e environment, and susceptibility to <strong>in</strong>fection. The need forassisted ventilation and supplemental oxygen <strong>in</strong> these <strong>in</strong>fants contributes to a form of chronic lung<strong>in</strong>jury <strong>in</strong>itially described by Northway et al. 4 as BPD. In recent years, with <strong>in</strong>creased survival of ELBWPremature lung with developmental deficiencies (surfactant, nitricoxide, immune defense, etc.) and immature alveoliHyperoxia Mechanical ventilationInfection Pulmonary edemaLung InjuryNeutrophil <strong>in</strong>flux Macrophage <strong>in</strong>fluxIncreased pro<strong>in</strong>flammatory cytok<strong>in</strong>esand altered growth factor productiongenetic susceptibilitySurfactant dysfunctionFailure of alveogenesisIncreased airway and vascular resistanceAbnormal repair (elast<strong>in</strong> deposition)BRONCHOPULMONARY DYSPLASIAFigure. Simplified schema of thepathogenesis of BPD and proposed roleof surfactant dysfunction. Lung <strong>in</strong>jury <strong>in</strong>the immature lung secondary tohyperoxia, mechanical ventilation and<strong>in</strong>fection <strong>in</strong>itiates an <strong>in</strong>flammatoryresponse and altered growth and<strong>in</strong>flammatory factor milieu. Oneresponse to these changes is decreasedSP-B/C content, which results <strong>in</strong> <strong>in</strong>activesurfactant. Loss of SP-B and surfactantfunction <strong>in</strong>creases lung susceptibility to<strong>in</strong>jury from both oxygen and <strong>in</strong>fection,promot<strong>in</strong>g and prolong<strong>in</strong>g the<strong>in</strong>flammatory and growth factorresponse. This cont<strong>in</strong>u<strong>in</strong>g <strong>in</strong>sultcontributes to the long-term changes <strong>in</strong>lung structure that characterize BPD.<strong>in</strong>fants, another form of BPD has been identified. This “new BPD” is characterized by impairedalveolar development with excess tone and reactivity of pulmonary arterial and airway smooth muscle5-8 . As described <strong>in</strong> the report validat<strong>in</strong>g the NIH consensus def<strong>in</strong>ition of BPD, severe BPD is def<strong>in</strong>edas a requirement for ventilatory support and/or supplemental oxygen >30% <strong>in</strong> an <strong>in</strong>fant at 36 wk PMA9 . The etiology of BPD is clearly multifactorial and <strong>in</strong>volves derangements <strong>in</strong> multiple aspects of lungfunction (e.g., surfactant production), repair from <strong>in</strong>jury (e.g. elast<strong>in</strong> deposition) and growth anddevelopment (e.g. alveologenesis). Various factors contribute to this process, <strong>in</strong>clud<strong>in</strong>g a susceptiblehost with immature lung structure, and developmental deficiencies of factors crucial to lungdevelopment and function such as surfactant, nitric oxide, <strong>in</strong>nate immune defense, and antioxidantcapability (Figure). It is quite unlikely that a s<strong>in</strong>gle therapy targeted at one component of thepathogenesis (e.g., iNO to improve lung development) will be sufficient to completely prevent BPD.Rather, a comb<strong>in</strong>ation of treatments directed at different aspects of the pathogenesis may beexpected to provide additive benefit.1.2 Epidemiology of BPD. There are approximately 29,000 <strong>in</strong>fants of 500-999 g birth weight (
emergency room visits, re-hospitalizations and long-term pulmonary disability (<strong>in</strong>clud<strong>in</strong>g asthma andsusceptibility to <strong>in</strong>fection), and <strong>in</strong>creased risk of late mortality and neurodevelopmental delay.1.3 Pulmonary surfactant. Pulmonary surfactant is a mixture of lipids and prote<strong>in</strong>s that provides thesurface-active alveolar film required for normal lung function and survival. The predom<strong>in</strong>ant surfactantphospholipid is disaturated phosphatidylchol<strong>in</strong>e (dipalmitoylphosphatidylchol<strong>in</strong>e, or DPPC), a highlycompressible lipid that provides a surface film at end-expiration, reduc<strong>in</strong>g surface tension to nearlyzero to prevent collapse of alveoli and term<strong>in</strong>al airways. Two of the surfactant-associated prote<strong>in</strong>s,SP-B and SP-C, are <strong>in</strong>timately associated with surfactant lipids and are crucial for surfactant filmformation and function <strong>in</strong> the lung. SP-B is also required for proper packag<strong>in</strong>g of surfactant <strong>in</strong> lamellarbodies with<strong>in</strong> type II respiratory epithelial cells 10 . Most ELGAN <strong>in</strong>fants have a developmentaldeficiency of surfactant at birth. Production of active surfactant, with normal levels of SP-B and SP-C,develops after birth but is delayed as long as three wk <strong>in</strong> some <strong>in</strong>fants 11 . Numerous cl<strong>in</strong>ical trials haveestablished the safety and efficacy of surfactant treatment of premature <strong>in</strong>fants immediately afterdelivery 12, 13 , with decreased mortality and <strong>in</strong>cidence of air leak syndromes.Most <strong>in</strong>fants who rema<strong>in</strong> <strong>in</strong>tubated beyond the first wk of life experience one or more episodes ofdysfunctional surfactant, def<strong>in</strong>ed as an abnormal m<strong>in</strong>imum surface tension value (>5 mN/m) <strong>in</strong> vitrofor surfactant isolated from tracheal aspirate samples. Moreover, episodes of dysfunctional surfactantare highly associated (p=0.005) with respiratory deteriorations and <strong>in</strong>creased requirement forventilatory support 1 . This observation provides the rationale for later doses of surfactant to preventepisodes of respiratory decompensation.A variety of commercial surfactant formulations are safe and effective when adm<strong>in</strong>istered topremature <strong>in</strong>fants at birth. For this cl<strong>in</strong>ical trial of late surfactant treatment we have selected Infasurf(ONY <strong>in</strong>c), a natural surfactant extracted from bov<strong>in</strong>e lung lavage fluid, which has consistent amountsof SP-B (0.9% phospholipid) and SP-C (1.5% phospholipid). Animal studies <strong>in</strong>dicate an important rolefor surfactant <strong>in</strong> prevention of lung <strong>in</strong>jury and resistance to <strong>in</strong>fection. Alterations <strong>in</strong> surfactant functionand/or SP concentrations have been reported <strong>in</strong> patients with Acute Respiratory Distress Syndrome(ARDS) 14 , pneumonia 15 , viral bronchiolitis 16 , adult chronic lung disorders 17 and Pneumocystis<strong>in</strong>fections 18 .To date, there are limited data evaluat<strong>in</strong>g surfactant therapy for premature <strong>in</strong>fants who requirecont<strong>in</strong>u<strong>in</strong>g ventilatory support beyond one wk of life. Pandit et al. 19 reported a study of 10 premature<strong>in</strong>fants, median gestational age 25 wk, at 9-30 d of life who were given a s<strong>in</strong>gle dose of acommercially available bov<strong>in</strong>e lipid extract surfactant. Median FiO 2 decreased significantly by 24 hand rema<strong>in</strong>ed significantly less than at study entry for 72 h. A report from Biss<strong>in</strong>ger et al. 20 alsodemonstrated a transient improvement <strong>in</strong> oxygenation of premature <strong>in</strong>fants >7 d of life with diffuselung disease or respiratory decompensation, after treatment with 2 doses of surfactant. Katz and Kle<strong>in</strong>21 reported a retrospective cohort study of 25 premature <strong>in</strong>fants, median gestational age of 24.7 wk,receiv<strong>in</strong>g booster surfactant treatment on median d 12 of life for worsen<strong>in</strong>g oxygenation. Thesurfactant treatment was well tolerated, and 70% of those treated had a short-term improvement <strong>in</strong>Respiratory Severity Score (RSS, mean airway pressure x FiO 2 ) after surfactant therapy.1.4 Pilot Trials. We have recently conducted two late Infasurf surfactant treatment pilot trials <strong>in</strong>premature <strong>in</strong>fants who were not receiv<strong>in</strong>g iNO. In the first pilot trial, “Pilot Trial of Surfactant BoosterProphylaxis For Ventilated Preterm Neonates
Infants <strong>in</strong> the historical control group had a significant, progressive, daily <strong>in</strong>crease <strong>in</strong> RespiratorySeverity Scores throughout the entire 2nd wk of life, as compared to basel<strong>in</strong>e (d 8) values. Infants <strong>in</strong>the surfactant pilot trial had an <strong>in</strong>itial improvement <strong>in</strong> respiratory severity scores after the firstsurfactant dose as compared to basel<strong>in</strong>e scores, but similar to historical controls, had significantlyhigher respiratory severity scores than basel<strong>in</strong>e by 5 d after <strong>in</strong>itial surfactant treatment. Of note, 65%of tracheal aspirate samples obta<strong>in</strong>ed before enrollment <strong>in</strong>to the surfactant pilot trial (n=17) hadabnormal surface tension, confirm<strong>in</strong>g the earlier f<strong>in</strong>d<strong>in</strong>g that surfactant dysfunction is common <strong>in</strong><strong>in</strong>fants requir<strong>in</strong>g mechanical ventilation after 7 d of age. The <strong>in</strong>cidence of abnormal m<strong>in</strong>imum surfacetension (>5 mN/m) the day after treatment was 38%. However by study d 3 and 7, 58% and 60% oftracheal aspirate samples obta<strong>in</strong>ed aga<strong>in</strong> had abnormal surface tension, suggest<strong>in</strong>g a loss <strong>in</strong> benefitat the third study day after <strong>in</strong>itial treatment.Based on these Respiratory Severity Score and tracheal aspirate data, a second pilot surfactantreplacement trial, “Pilot Trial of Surfactant Booster Prophylaxis For Ventilated Preterm Neonates Less
NO is required for normal lung development, that NO production is deficient after premature birth, andthat replacement iNO therapy, over a period of weeks, is beneficial <strong>in</strong> the <strong>in</strong>jured lung.1.5.b. Cl<strong>in</strong>ical Trials. iNO is established as an effective treatment for persistent pulmonaryhypertension occurr<strong>in</strong>g <strong>in</strong> term newborn <strong>in</strong>fants 29-31 . The success of iNO treatment of the term <strong>in</strong>fantled to studies of iNO treatment <strong>in</strong> the preterm <strong>in</strong>fant that have focused on either 1) treatment ofcritically ill <strong>in</strong>fants at the time of birth with the hypothesis that treatment might result <strong>in</strong> improvedsurvival 32-34 , or 2) treatment of preterm <strong>in</strong>fants with established BPD 35, 36 or at high risk of BPD 2, 37, 38 .1.5.c. Prevention of BPD with the use of <strong>in</strong>haled Nitric Oxide (iNO): the NO CLD Trial. We recentlypublished the results of our multicenter randomized cl<strong>in</strong>ical trial of iNO to prevent BPD <strong>in</strong> high riskpreterm <strong>in</strong>fants (2,3) The trial was conducted between May, 2000 and April, 2005 at 21 NICUs <strong>in</strong> 14cities across the United States and <strong>in</strong>volved <strong>in</strong>fants of birth weights between 500 and 1250 g whocont<strong>in</strong>ued to require ventilatory support between 7 and 21 d of age. The meanSD birth weight of the<strong>in</strong>fants enrolled was 766±161 g <strong>in</strong> the treated group and 759±155 g <strong>in</strong> the placebo group. There wasno difference between the treatment groups <strong>in</strong> gestational age, racial background, number whoreceived antenatal glucocorticoids, surfactant at birth, or vitam<strong>in</strong> A. The primary outcome <strong>in</strong> that trialwas survival without BPD at 36 wk PMA. For the <strong>in</strong>fants enrolled between 7 and 14 d (as will occur <strong>in</strong>the proposed surfactant trial), the rate of survival without BPD at 36 wk PMA was 49% <strong>in</strong> the treatedgroup and 27% <strong>in</strong> the placebo group (p=0.005). The relative benefit of treatment was 1.91(1.31-2.78)with NNT =4. In that trial (as is proposed <strong>in</strong> TOLSURF) all <strong>in</strong>fants from a given pregnancy, althoughassigned a randomization number, received the same therapy. This required biostatistical analysistechniques (GEE and Multiple Outputation) that take <strong>in</strong>to account cluster<strong>in</strong>g 40 .Inhaled nitric oxide treatment <strong>in</strong> this group also shortened duration of both hospitalization (bymean of 9 d), ventilatory support (by mean of 7 d), and fewer <strong>in</strong>fants were discharged onsupplemental oxygen therapy. A cost effectiveness analysis done by John Zupancic 41 found thatthere was an average sav<strong>in</strong>g of ~$5,000 for <strong>in</strong>fants treated with iNO between 7 and 14 d. We saw nocl<strong>in</strong>ical or laboratory evidence of short-term adverse effects. In evaluation of the one-year pulmonaryoutcome of this group of <strong>in</strong>fants; there was a significant decrease <strong>in</strong> the number of children whoreceived <strong>in</strong>haled steroids, supplemental oxygen or bronchodilators 42 . We found no change <strong>in</strong> the<strong>in</strong>cidence of standard neonatal co-morbidities, namely <strong>in</strong>fection, PDA, NEC, extension of IVH/PVL, orROP, and no elevation of methemoglob<strong>in</strong> levels <strong>in</strong> any of the iNO-treated <strong>in</strong>fants. The long-termneurodevelopmental outcome data from the NO CLD trial <strong>in</strong>dicate that there is no deleterious43, 44outcome related to iNO treatment We believe that the results of the NO CLD Trial, along with thef<strong>in</strong>d<strong>in</strong>gs <strong>in</strong> other trials and the biological plausibility demonstrated <strong>in</strong> animal studies, <strong>in</strong>dicate that iNOis an important treatment modality for reduc<strong>in</strong>g the <strong>in</strong>cidence and severity of BPD and has becomeaccepted therapy for prevention of BPD <strong>in</strong> many NICUs.1.5d.NIH Consensus Conference on iNO <strong>in</strong> the Preterm Infant. A Consensus Conference washeld <strong>in</strong> Bethesda on Oct 27-29, 2010. The panel drew the follow<strong>in</strong>g conclusion:“ Taken as a whole, the available evidence does not support use of iNO <strong>in</strong> early rout<strong>in</strong>e, earlyrescue, or later rescue regimens <strong>in</strong> the care of premature <strong>in</strong>fants
evaluat<strong>in</strong>g the charges for iNO that might be passed on to the families. INO will be provided at nocharge by IKARIA to <strong>in</strong>fants enrolled <strong>in</strong> the trial.1.6 Interaction Between Surfactant And Nitric Oxide. We plan to study the addition of late surfactantadm<strong>in</strong>istration to <strong>in</strong>fants at high risk for BPD who are receiv<strong>in</strong>g iNO therapy. To our knowledge therehas been only one published study <strong>in</strong> animals address<strong>in</strong>g the potential <strong>in</strong>teraction of the 2 treatments.A study <strong>in</strong> piglets exam<strong>in</strong>ed responses to iNO and surfactant with acute lung <strong>in</strong>jury secondary to E.coli sepsis 45 . Both treatments improved the respiratory status at 24 h compared to control, andcomb<strong>in</strong>ed therapy provided the best outcome. These f<strong>in</strong>d<strong>in</strong>gs support the safety of comb<strong>in</strong>ed iNOand surfactant treatment and suggest additive benefit <strong>in</strong> this experimental model.A number of studies have exam<strong>in</strong>ed effects of iNO on endogenous surfactant. In many cell cultureand animal studies, which used relatively high doses of NO, exposure reduced surfactant recovery or<strong>in</strong>hibited surfactant function 46-52 . More relevant, chronic exposure of <strong>in</strong>fant baboons to a cl<strong>in</strong>icallyrelevant dose of iNO had beneficial effects on surfactant. iNO-treated animals had improvedsurfactant phospholipid/prote<strong>in</strong> ratio and efficiency of SP-B/-C to promote low surface tension was<strong>in</strong>creased 39 .Because of reported negative effects of iNO on surfactant <strong>in</strong> some studies, as well as thevulnerability of the surfactant system <strong>in</strong> preterm <strong>in</strong>fants, we prospectively exam<strong>in</strong>ed surfactantfunction and composition as part of the NO CLD Trial. We collected tracheal aspirates from asubpopulation of study <strong>in</strong>fants and determ<strong>in</strong>ed effects of iNO on recovery of surfactant as well assurface tension properties. Aspirate samples were collected at study entry (just prior to receiv<strong>in</strong>gstudy gas) and at 24-48 h, 4 d, and then weekly after <strong>in</strong>itiation of study gas until extubation.Surfactant function data were obta<strong>in</strong>ed at study entry for 83 <strong>in</strong>fants (41 iNO-treated <strong>in</strong>fants and 42placebo <strong>in</strong>fants). The mean gestational age (25.5 wk), birth weight (725-755 g) and racial distributionwere similar for the two groups. The mean age at enrollment was 15 d for both groups and theseverity of lung disease at enrollment, as assessed by the RSS, was similar between groups. The<strong>in</strong>fants <strong>in</strong> this study were representative of the entire population of <strong>in</strong>fants <strong>in</strong> the NO CLD Trial withregard to primary outcome and the demographic factors 53 .Values for m<strong>in</strong>imum surface tension were obta<strong>in</strong>ed by pulsat<strong>in</strong>g bubble surfactometer; thismeasurement reflects the ability of surfactant to form a stable surface film with a normal value form<strong>in</strong>imum surface tension of 5 mN/m, measured while the <strong>in</strong>fant rema<strong>in</strong>ed <strong>in</strong>tubated after <strong>in</strong>itiation of studygas. Data for this analysis were available for 38 <strong>in</strong>fants <strong>in</strong> each group for a mean duration of 3.9 and3.1 wk (p=0.03) after trial entry for control and treated <strong>in</strong>fants, respectively. The mean percent ofsamples taken over time while <strong>in</strong>tubated that had dysfunctional surfactant was 53.4% for control<strong>in</strong>fants and 48.1% for treated <strong>in</strong>fants (NS). Thus, despite an <strong>in</strong>itial lower<strong>in</strong>g of m<strong>in</strong>imum surfacetension after beg<strong>in</strong>n<strong>in</strong>g iNO, treated <strong>in</strong>fants cont<strong>in</strong>ued to experience episodes of dysfunctionalsurfactant dur<strong>in</strong>g the time they rema<strong>in</strong>ed <strong>in</strong>tubated. This f<strong>in</strong>d<strong>in</strong>g supports our proposal that <strong>in</strong>fants oniNO might benefit from later doses of surfactant to prevent or ameliorate the respiratory deteriorationsaccompany<strong>in</strong>g surfactant dysfunction.We also evaluated the relationship between surface tension and the primary cl<strong>in</strong>ical outcome <strong>in</strong>the trial. Survival without BPD <strong>in</strong> the iNO-treated group was 60% for <strong>in</strong>fants who had a decrease <strong>in</strong>m<strong>in</strong>imum surface tension between d 3-7 after study entry compared to 25% for those <strong>in</strong>fants withoutimproved surfactant function. In the placebo group, 25% of <strong>in</strong>fants who had a decrease <strong>in</strong> m<strong>in</strong>imumsurface tension had a favorable outcome compared to 53% of the <strong>in</strong>fants without improved surfactantfunction. None of these differences were statistically significant, although the study was notsufficiently powered to address this question.1/18/2011 11
2.0 HYPOTHESIS AND SPECIFIC AIMS2.1 Hypothesis and Rationale for TrialIn this trial, we hypothesize that late doses of surfactant, <strong>in</strong> addition to iNO, adm<strong>in</strong>istered to ExtremelyLow Gestational Age <strong>Newborn</strong> (ELGAN) <strong>in</strong>fants
2.2 Specific AimsAim 1. Assess the effect of late doses of surfactant <strong>in</strong> ventilated ELGANS receiv<strong>in</strong>g <strong>in</strong>halednitric oxide on survival without BPD.We will conduct a multi-center placebo-controlled trial of late surfactant treatment <strong>in</strong> addition toiNO <strong>in</strong> <strong>in</strong>fants at high risk of BPD. We will enroll 524 <strong>in</strong>fants
the trial, a DNA repository will be developed for analysis of specific gene polymorphisms <strong>in</strong> separatelyfunded <strong>in</strong>itiatives.3.0 RESEARCH DESIGNWe will perform a randomized, bl<strong>in</strong>ded, multicenter, placebo-controlled trial of late surfactanttreatment adm<strong>in</strong>istered to ventilated preterm <strong>in</strong>fants < 28weeks gestation beg<strong>in</strong>n<strong>in</strong>g between 7 and 14days of age. Randomization will be stratified by site and gestational age at birth. (< 26 weeks or 26 to28 weeks GA).3.1 Sites Involved. Subjects will <strong>in</strong>itially be recruited from eligible <strong>in</strong>patients with<strong>in</strong> the NeonatalIntensive Care Units listed <strong>in</strong> Appendix A section 23.0Study sample size is 524 patients. There is no enrollment restriction based on gender, ethnicity, orrace. Enrollment is expected to take about 38 to 40 months plus follow-up through 22 - 26 monthscorrected age.3.2 Site Activities. See Table for tim<strong>in</strong>g of procedures and data and sample collection. All sites will:- Screen <strong>in</strong>fants ≤28 w/0 d GA for eligibility and enrollment- Collect cl<strong>in</strong>ical data on pulmonary course and outcome- Assess the <strong>in</strong>cidence of common co-morbidities of preterm <strong>in</strong>fants <strong>in</strong>clud<strong>in</strong>g<strong>in</strong>fection, <strong>in</strong>tracranial hemorrhage, necrotiz<strong>in</strong>g enterocolitis and ret<strong>in</strong>opathy ofprematurity.- Collect samples of tracheal aspirates and ur<strong>in</strong>e at specified times for analysis ofsurfactant function and <strong>in</strong>flammatory markers as well as biomarkers of long termpulmonary disease- Evaluate long-term pulmonary and neurodevelopment outcome through 22 -26 monthsof age.TABLE 3.1STUDY PROCEDURE CHARTStudyDay/CorrectedAgePre-Study0 2 b 4 b 6 b 8 b 36wks40wks3, 6 &9 mos12mos22 - 26mosInformedconsentXRandomizationXTrachealX X X X XAspirate aStudySurfactant orX X X X Xprocedure ashamUr<strong>in</strong>e sample c X X X X XOxygenreductionchallengeXX1/18/2011 14
RespiratorystatusquestionnaireX(DC)X X X (18mos)NeurodevelopmentalevaluationXXa If rema<strong>in</strong>s <strong>in</strong>tubated and mechanically ventilatedb ± 24 hc Ur<strong>in</strong>e will be collected prior to iNO (if possible) and/or twice <strong>in</strong> week 1 and then weekly to 36 wkPMA4.0 ELIGIBILITY4.1. Inclusion Criteria- < 28 0/7 wk gestational age- Day of life 7-14- Intubated and mechanically ventilated- Plan to treat with iNO4.2. Exclusion Criteria- Serious congenital malformations or chromosomal abnormality (see MOP)- Life expectancy 24 hours4. Acute NEC –less than 24 hours from diagnosis or surgery5. Untreated culture positive sepsis (147. Cl<strong>in</strong>ical team feels <strong>in</strong>fant would not tolerate dos<strong>in</strong>g procedureNOTE: Once these issues resolve the <strong>in</strong>fant may be considered either for enrollment <strong>in</strong> TOLSURFwith <strong>in</strong>itial dos<strong>in</strong>g or for re-dos<strong>in</strong>g if dos<strong>in</strong>g was delayed5.0 RECRUITMENT AND RANDOMIZATION5.1 Recruitment. To facilitate timely enrollment <strong>in</strong> the trial, <strong>in</strong>fants < 28 w GA will be screened at age1-4d by the study physicians and nurses for eligibility and exclusion criteria and a brochure expla<strong>in</strong><strong>in</strong>gthe study <strong>in</strong> lay language will be provided to the family. Records of potential candidates for the studywill then be reviewed by one of the study <strong>in</strong>vestigators and the child’s condition discussed with theattend<strong>in</strong>g physician. If appropriate, the <strong>in</strong>fant’s parents will be approached by one of the <strong>in</strong>vestigatorsto obta<strong>in</strong> <strong>in</strong>formed consent and provide the Information Sheet about iNO <strong>in</strong> the Preterm Infant -preferably with<strong>in</strong> the first 3 days with the expectation that if the <strong>in</strong>fant meets criteria between 7 and 14d of age, he/she will be randomized and enrolled at that time. It is expected that there will be a1/18/2011 15
easonably high acceptance rate by parents for participation <strong>in</strong> this study because a) these <strong>in</strong>fants, ifstill requir<strong>in</strong>g ventilation between 7 and 14 d, have at best a 44% chance of survival without BPD at 36wk PMA with usual therapy (<strong>in</strong>clud<strong>in</strong>g iNO) alone; b) late treatment with surfactant (Infasurf)represents extra doses of a substance (surfactant) that their <strong>in</strong>fant received at birth as part ofstandard cl<strong>in</strong>ical care. Infants who are still <strong>in</strong>tubated or re<strong>in</strong>tubated between d 7 -14 will be enrolled <strong>in</strong>the trial, begun on iNO, and randomized to receive surfactant study drug or sham placebo treatment.5.2 Accrual - Meet<strong>in</strong>g Recruitment Targets. We have chosen sites for this trial that can be expected toenroll a m<strong>in</strong>imum of 8 to 10 <strong>in</strong>fants/year <strong>in</strong> the study (many expect to enroll more than 15/year).We plan to recruit at 16 sites (4 of which have two hospitals) Based on our experience to date withthe TOLSURF Pilot study, we anticipate that the participat<strong>in</strong>g sites will meet their enrollment targetsand that subject accrual will follow the projected timel<strong>in</strong>e. We plan 6 months (from 9/15/09) forf<strong>in</strong>aliz<strong>in</strong>g preparations to beg<strong>in</strong> enrollment <strong>in</strong> the study. We expect that the 7 sites (9 hospitals)<strong>in</strong>volved <strong>in</strong> the pilot will be able to beg<strong>in</strong> enroll<strong>in</strong>g by March, 2010 (averag<strong>in</strong>g 5 <strong>in</strong>fants/month). Weexpect 3 additional sites(4 hospitals) to beg<strong>in</strong> enroll<strong>in</strong>g by mid June (<strong>in</strong>creas<strong>in</strong>g the monthlyenrollment for the 10 <strong>in</strong>itial sites to 8/mo and an estimated 39 <strong>in</strong>fants <strong>in</strong> the first 6 mos - 9/15/10). Weexpect that another 2-3 sites will be ready to enroll by 9/15/10 and the f<strong>in</strong>al 3 ready by 12/15/09 toreach the goal of enroll<strong>in</strong>g 11/month by the end of the second 6 mo and 16/mo <strong>in</strong> year 2 and 3. Thisprojection would lead to 111 <strong>in</strong>fants <strong>in</strong> year one (by 3/15/11), additional 192 <strong>in</strong> year 2 (303 total) and192 <strong>in</strong> year 3 (495 total) expect<strong>in</strong>g to complete enrollment <strong>in</strong> 38-39 mo. However, we recognize thatsome sites might fail to meet enrollment targets for a variety of reasons. Accord<strong>in</strong>gly, we will assesssite enrollment every 6 mo after <strong>in</strong>itiation of the study. Should enrollment at an <strong>in</strong>dividual site fallbelow 4 <strong>in</strong> 6 mo, the PI and Cl<strong>in</strong>ical Steer<strong>in</strong>g Committee will give warn<strong>in</strong>g and evaluate whether it isappropriate to drop the site from the trial. If no patients are entered dur<strong>in</strong>g a 6 mo period we willrecommend to the DSMB that the site be dropped and a new site recruited. If a s<strong>in</strong>gle site appears tobe enroll<strong>in</strong>g more than 20% of the total <strong>in</strong>fants enrolled <strong>in</strong> the trial we will temporarily limit enrollmentat that site.5.3 Randomization. Randomization tables will be prepared by the DCC and sent to the Pharmacist atthe sites. This study has been designed to allow for randomization 8 AM to 5 PM dur<strong>in</strong>g week days.The site will phone the Project Director and confirm that the <strong>in</strong>fant meets the eligibility criteria anddeterm<strong>in</strong>e if the <strong>in</strong>fant is part of a multiple birth. If eligibility is confirmed, a study ID number will beassigned to the patient and <strong>in</strong>structions given to the site to enroll the <strong>in</strong>fant. Tracheal Aspirate andur<strong>in</strong>e samples will be obta<strong>in</strong>ed. The physician will write the order to RRT to beg<strong>in</strong> iNO and to thepharmacy to assign the next treatment to the <strong>in</strong>fant. The Project Director will complete an entry onher log giv<strong>in</strong>g the date, time, patient ID, confirmation number, etc. These can later be confirmedaga<strong>in</strong>st the cl<strong>in</strong>ical center records. Only the pharmacist will be unbl<strong>in</strong>ded to treatment assignment.Patients will be stratified by gestational age (
ppm and 2 ppm at weekly <strong>in</strong>tervals for a total m<strong>in</strong>imum treatment of 25 d. Gas will be cont<strong>in</strong>ued byNCPAP or nasal cannula if the <strong>in</strong>fant is extubated. INO will be provided at no charge by IKARIA, Inc.6.2 Late Surfactant Treatment. Infasurf (ONY Inc., Amherst, NY) is an FDA-approved, commerciallyavailable extract of bov<strong>in</strong>e natural surfactant, which conta<strong>in</strong>s phospholipids, neutral lipids and aconsistent concentration of SP-B and SP-C (0.9% SP-B to phosphatidylchol<strong>in</strong>e ratio). The efficacy ofInfasurf at birth has been demonstrated <strong>in</strong> multiple trials and this formulation will be used for thestudy surfactant at all study sites. The lot number for each dose of Infasurf will be recorded.Infasurf will be provided at no charge by ONY, Inc.6.3 Instillation Procedure. Infants must be considered cl<strong>in</strong>ically stable at the time of enrollment (seeTable 4.3 and MOP for def<strong>in</strong>ition). Either Infasurf surfactant or a sham treatment is adm<strong>in</strong>istered assoon as possible after the <strong>in</strong>itiation of iNO, us<strong>in</strong>g standard procedures and precautions as for newborntreatment. In order to ma<strong>in</strong>ta<strong>in</strong> bl<strong>in</strong>d<strong>in</strong>g, screens are set around the bedside, shield<strong>in</strong>g the <strong>in</strong>fant frompersonnel <strong>in</strong> the room. Monitors are on and active, but silenced. Endotracheal tube (ETT) patency andappropriate position should be assured prior to <strong>in</strong>still<strong>in</strong>g study drug. Study drug is usuallyadm<strong>in</strong>istered by one or more respiratory therapists who are tra<strong>in</strong>ed <strong>in</strong> the <strong>in</strong>stillation procedure. Othermembers of the health-care team step away from the bedside dur<strong>in</strong>g study drug <strong>in</strong>stillation. An<strong>in</strong>dividual skilled <strong>in</strong> <strong>in</strong>tubation must be present <strong>in</strong> the nursery at the time of study drug <strong>in</strong>stillation <strong>in</strong>case of dislodg<strong>in</strong>g or plugg<strong>in</strong>g of the endotracheal tube requir<strong>in</strong>g re-<strong>in</strong>tubation. There will be nomanipulation of the ETT of <strong>in</strong>fants <strong>in</strong> the sham treatment group. Study drug is adm<strong>in</strong>istered per the<strong>in</strong>stitution’s regular surfactant adm<strong>in</strong>istration policy and care is taken to ma<strong>in</strong>ta<strong>in</strong> lung <strong>in</strong>flation dur<strong>in</strong>g<strong>in</strong>stillation. Ventilator adjustment guidel<strong>in</strong>es will be provided to address the response to anysignificant changes <strong>in</strong> oxygenation, ventilation, heart rate or chest wall movement dur<strong>in</strong>g or after theprocedure. The dos<strong>in</strong>g respiratory therapists will attempt to wean the ventilatory support back tobasel<strong>in</strong>e sett<strong>in</strong>gs over a 20 m<strong>in</strong> period, after which the audible alarms are reset and the screens areremoved. Vital signs, saturations, severity score and ventilator sett<strong>in</strong>gs will be recorded prior to and at60 and 120 m<strong>in</strong>utes after each study procedure. Subsequent ventilatory management is at thediscretion of the cl<strong>in</strong>ical medical team, with a common ventilator strategy to ma<strong>in</strong>ta<strong>in</strong> adequate lung<strong>in</strong>flation and m<strong>in</strong>imize overdistention and oxygen exposure. See the MOP for a detailed description ofthe procedure.6.4 Bl<strong>in</strong>d<strong>in</strong>g of Surfactant/Placebo Adm<strong>in</strong>istration. The study drug, prepared by the pharmacist, is 3ml/kg Infasurf surfactant or an equivalent volume of air as placebo; the air is not adm<strong>in</strong>istered. Thedrug is drawn up <strong>in</strong> two half doses (1.5 ml/kg) and sent to the nursery <strong>in</strong> syr<strong>in</strong>ges covered <strong>in</strong> anopaque envelope to ma<strong>in</strong>ta<strong>in</strong> bl<strong>in</strong>d<strong>in</strong>g. All parents, study and cl<strong>in</strong>ical staff, except the “dos<strong>in</strong>g”respiratory therapist and the research pharmacist are bl<strong>in</strong>ded to treatment assignment. Cl<strong>in</strong>icalrespiratory therapy staff, but not the “dos<strong>in</strong>g” therapist, may make suggestions about ventilatormanipulations <strong>in</strong> study <strong>in</strong>fants immediately after study drug delivery. Every attempt will be made tolimit unbl<strong>in</strong>d<strong>in</strong>g of study subject assignment. Staff <strong>in</strong>volved <strong>in</strong> the dos<strong>in</strong>g procedure will collect<strong>in</strong>formation about how the <strong>in</strong>fant tolerates dos<strong>in</strong>g (bradycardia,desaturation or re<strong>in</strong>tubation) and fax tothe DCC separate from the rest of the CRF. They will not be <strong>in</strong>volved <strong>in</strong> any other aspect of datacollection, determ<strong>in</strong>ation of the endpo<strong>in</strong>t at 36 or 40 weeks – or adm<strong>in</strong>ister<strong>in</strong>g the O2/flow challengetest. We have previous experience with randomization <strong>in</strong> the pharmacy 54 and unbl<strong>in</strong>dedadm<strong>in</strong>istration of treatment by a respiratory therapist (NO CLD Trial). In neither trial was there an<strong>in</strong>stance of unbl<strong>in</strong>d<strong>in</strong>g of the PI, and several of the trial centers also have experience with bl<strong>in</strong>d<strong>in</strong>g ofsurfactant treatment <strong>in</strong> the manner proposed.6.5 Retreatment with Surfactant/Placebo. If the <strong>in</strong>fant rema<strong>in</strong>s <strong>in</strong>tubated 1-3 days after the first studydrug treatment, a second dose of the same assigned study treatment is adm<strong>in</strong>istered us<strong>in</strong>g the sameprocedure. Up to 3 additional doses of study drug (total 5 doses) may be given at 1-3d <strong>in</strong>tervals if the<strong>in</strong>fant rema<strong>in</strong>s <strong>in</strong>tubated. Retreatment <strong>in</strong>tervals after the first dose for this trial are based uponrespiratory severity score data and tracheal sample analysis from the first three surfactant pilot trials.This treatment schedule will provide active surfactant for <strong>in</strong>fants <strong>in</strong>to the third or fourth wk of life. Wehave found that the highest <strong>in</strong>cidence of respiratory deterioration associated with surfactant1/18/2011 17
dysfunction occurs dur<strong>in</strong>g the postnatal <strong>in</strong>terval from 7 to 28 d 1 . Should the <strong>in</strong>fant be consideredcl<strong>in</strong>ically unstable by the physicians at the time when a dose (see Table 4.3 and as def<strong>in</strong>ed <strong>in</strong> theMOP) should be given, but later stabilizes dur<strong>in</strong>g the study treatment period, the <strong>in</strong>fant will cont<strong>in</strong>uewith study drug dos<strong>in</strong>g as per the dos<strong>in</strong>g schedule outl<strong>in</strong>ed above, and is still eligible for a total of 5study doses dur<strong>in</strong>g the 3 weeks after enrollment.6.6 Patients Extubated with<strong>in</strong> 24 Days of Study Initiation. Patients who are extubated between StudyDay 1 and Study Day 25 will cont<strong>in</strong>ue on iNO, but will not have subsequent tracheal aspiratesobta<strong>in</strong>ed, and will not receive subsequent surfactant/placebo doses. However, if an <strong>in</strong>fant is re<strong>in</strong>tubated,the patient will have subsequent tracheal aspirates obta<strong>in</strong>ed, and will receivesurfactant/placebo, accord<strong>in</strong>g to the study schedule. For <strong>in</strong>fants who are extubated, and then requirere-<strong>in</strong>tubation, a tracheal aspirate sample will be obta<strong>in</strong>ed with<strong>in</strong> 24 h of re<strong>in</strong>tubation, and trachealaspirate sampl<strong>in</strong>g will then cont<strong>in</strong>ue as outl<strong>in</strong>ed above while the <strong>in</strong>fant is receiv<strong>in</strong>g study drug.7.0 STUDY TREATMENT MODIFICATIONS7.1 Indications for Interruption of Study Drug Adm<strong>in</strong>istration If <strong>in</strong>fant is deemed cl<strong>in</strong>ically unstable(Table 4.3) by the attend<strong>in</strong>g physician (<strong>in</strong>clud<strong>in</strong>g RSS > 14 for > 8 hours or as otherwise def<strong>in</strong>ed <strong>in</strong> theMOP) dos<strong>in</strong>g will be delayed until RSS is < 14 and <strong>in</strong>fant considered stable by the cl<strong>in</strong>ical team.7.2 Stopp<strong>in</strong>g Study Drug Adm<strong>in</strong>istration for Individual Patient. If the <strong>in</strong>fant experiences severerespiratory decompensation immediately after the study drug adm<strong>in</strong>istration procedure with asusta<strong>in</strong>ed (for 24 h or more) change <strong>in</strong> RSS above basel<strong>in</strong>e of > 8 no further doses will be given butdata and sample collection will cont<strong>in</strong>ue.7.3 Open Label Use of Late Surfactant. If the attend<strong>in</strong>g physician deems that the <strong>in</strong>fant would benefitfrom late doses of surfactant the follow<strong>in</strong>g procedure is to be followed:- The decision must be approved by the site PI and reported to the CCC with<strong>in</strong> 72 h- Surfactant will not be furnished free for this use- DO NOT UNBLIND – cont<strong>in</strong>ue the <strong>protocol</strong> as appropriate- Data and sample collection and AE report<strong>in</strong>g on <strong>in</strong>fants treated Open Label should cont<strong>in</strong>ueas per study <strong>protocol</strong>.- A Protocol violation form must be filed8.0 OUTCOME MEASURES1/18/2011 18
8.1 Primary Outcome. The primary outcome issurvival without BPD at 36 wk PMA. Thisdef<strong>in</strong>ition is currently used as the primaryoutcome for other trials related to premature<strong>in</strong>fant lung disease. BPD is diagnosed, as <strong>in</strong>our NO CLD Trial, by a requirement at 36 wkPMA for ventilation and/or supplementaloxygen determ<strong>in</strong>ed by a room air challenge testfor those <strong>in</strong>fants still receiv<strong>in</strong>g oxygen. Infantsat 36 wk PMA who require mechanicalventilation and any level of supplementaloxygen, or oxygen >30% without assistedventilation, are diagnosed with BPD andconsidered “severe” BPD by the NIHConsensus def<strong>in</strong>ition 9 . Infants at 36 wk PMAon
and could have unforeseen effects on neurodevelopment. Thus, there is a strong rationale for acomprehensive outcome assessment program. All of the centers <strong>in</strong>volved <strong>in</strong> this trial have activeneonatal developmental follow-up programs. As <strong>in</strong> the NO CLD trial, all the sites will have a “GoldStandard” Bayley III exam<strong>in</strong>er for assessment at age 22 -26 mo. The MOP provides a description ofthe assessments to be done as well as the “Gold Standard” certification process. The early detectionof impaired survivors allows implementation of <strong>in</strong>tervention services, which may reduce the burden ofdisability <strong>in</strong> the survivors. We fully acknowledge the limitations of these early evaluations for theprediction of future <strong>in</strong>tellectual and school performance. However, if late surfactant is efficacious <strong>in</strong>improv<strong>in</strong>g survival without BPD, but survivors demonstrated more pronounced earlyneurodevelopmental abnormalities, it would be important to have this follow-up <strong>in</strong>formation before thistherapy became widely used. We do not expect late surfactant to have a deleterious effect onoutcome.9.0 DATA COLLECTION AND EVALUATION9.1 Cl<strong>in</strong>ical Data Collection. The cl<strong>in</strong>ical data <strong>in</strong>cludes details about respiratory support, developmentof BPD, selected medications, and significant co-morbidities. See the CRFs. The respiratory data<strong>in</strong>cludes the type of respiratory support, FiO2, and ventilator sett<strong>in</strong>gs, which will be used to calculatedaily respiratory severity scores (RSS = MAP X FiO2). Other respiratory outcomes <strong>in</strong>clude the lengthof ventilatory support, duration of oxygen supplementation, and hospitalization. BPD will be diagnosedat 36 wk PMA us<strong>in</strong>g the oxygen/flow challenge test similar as employed <strong>in</strong> the NO CLD trial.Respiratory status at 40 wk PMA will be assessed us<strong>in</strong>g the same oxygen challenge test whenappropriate. Data on medication adm<strong>in</strong>istration, <strong>in</strong>clud<strong>in</strong>g use of steroids, vitam<strong>in</strong> A, caffe<strong>in</strong>e,antibiotics and <strong>in</strong>domethac<strong>in</strong> will be collected. The <strong>in</strong>cidence of co-morbidities related to prematurityand other adverse events potentially related to tracheal aspirate sampl<strong>in</strong>g or adm<strong>in</strong>istration ofsurfactant will be recorded. Infants will be followed through 22- 26 mo of age for pulmonary andneurodevelopmental outcomes. Standardized pulmonary questionnaires will be adm<strong>in</strong>istered (usuallyby phone) every 3-4 mo until 1 y of age and aga<strong>in</strong> at 22 - 26 m of age <strong>in</strong> order to collect <strong>in</strong>formationabout respiratory status, ER visits and hospitalizations and pulmonary medications. See list of<strong>in</strong>formation to be collected <strong>in</strong> MOP.9.2 Other Cl<strong>in</strong>ical Management. Other therapies for treatment of BPD, <strong>in</strong> particular, postnatalglucocorticoid treatment, will be adm<strong>in</strong>istered accord<strong>in</strong>g to cl<strong>in</strong>ical management guidel<strong>in</strong>es agreedupon by all <strong>in</strong>vestigators, as was the case with the NO CLD Trial.(see Appendix B for guidel<strong>in</strong>es) Aspart of the education and site <strong>in</strong>itiation visit, the PI will discuss cl<strong>in</strong>ical <strong>protocol</strong> guidel<strong>in</strong>es with theattend<strong>in</strong>g neonatologists at that site. The <strong>protocol</strong>s <strong>in</strong>clude, but are not restricted to, the use ofsurfactant at birth, treatment with caffe<strong>in</strong>e, Vitam<strong>in</strong> A use, ventilator management and respiratory gasparameters, oxygen saturation targets, the use of NCPAP and high flow nasal cannula, the use ofcorticosteroids both for BPD and hypotension, as well as rescue treatment (open label) with surfactantfor severe lung disease. The <strong>in</strong>vestigator steer<strong>in</strong>g committee will review these guidel<strong>in</strong>es <strong>in</strong> light ofcurrent practice on at least an annual basis, and the sites will be monitored for compliance by staffdur<strong>in</strong>g site monitor<strong>in</strong>g visits.9.3 Data Quality Assurance. We will monitor the accuracy of data entry by the sites both <strong>in</strong>ternally andexternally. For <strong>in</strong>ternal monitor<strong>in</strong>g, completed CRFs are reviewed prior to entry <strong>in</strong>to the database,clarify<strong>in</strong>g issues as necessary with site coord<strong>in</strong>ators and act<strong>in</strong>g as liaison between the sites and theDCC as needed. These steps are already <strong>in</strong> place and function<strong>in</strong>g <strong>in</strong> the TOLSURF Pilot Study.External monitor<strong>in</strong>g will consist of regular monitor<strong>in</strong>g visits to every site while actively enroll<strong>in</strong>g. Initialmonitor<strong>in</strong>g visits will take place after the first 6-8 patients have completed data collection and thenafter every 10 to 15 <strong>in</strong>fants are subsequently enrolled and CRFs completed. All medical charts will bemonitored and compared to the CRFs for meet<strong>in</strong>g entry criteria, adherence to <strong>protocol</strong>, primary andsecondary outcomes, and adverse event report<strong>in</strong>g. In addition, a 25% random sample of all datapo<strong>in</strong>ts <strong>in</strong> the CRFs will be compared with the medical record. Any outstand<strong>in</strong>g data queries will beresolved with the research coord<strong>in</strong>ator at the time of the visit. After each study site visit a report will1/18/2011 20
e prepared and copies sent to the Study File, the DCC, the study PI (R. Ballard), the site PI, the sitecoord<strong>in</strong>ator and the Project Director (N. Newton).As part of the overall QA effort, we will exam<strong>in</strong>e various measures of study implementation acrosssites. In particular, recruitment, retention, data completeness, and measurement precision will betabulated and compared across sites and will be <strong>in</strong>cluded <strong>in</strong> our web-based reports. QA efforts andsite visits will be focused on any sites that show evidence of problems.10.0 SAMPLES AND LABORATORY STUDIES10.1 Tracheal Aspirate Samples.Each tracheal aspirate sample is centrifuged soon after collection to provide a cell pellet and asupernatant fraction. After shipment to UCSF, the supernatant is centrifuged to isolate a largeaggregate surfactant fraction that is assayed as previously described 1 for both phospholipid andsurfactant prote<strong>in</strong> concentration under the direction of Dr. Philip Ballard. The supernatant fractionafter surfactant isolation is assayed for selected cytok<strong>in</strong>es us<strong>in</strong>g a multiplex assay. The TA cell pelletis used for isolation of DNA to develop a repository for genetic studies of gene variants. All trachealaspirate samples are labeled by study ID number and date/time, without other identifiers. Should anyportion of sample rema<strong>in</strong>, we request that it will be stored, labeled only by study number, <strong>in</strong> thelaboratory of Dr. P. Ballard for further test<strong>in</strong>g of substances identified as related to airway<strong>in</strong>flammation and the development of BPD (this is addressed specifically <strong>in</strong> the consent form).Each tracheal aspirate sample is centrifuged soon after collection to provide a cell pellet and asupernatant fraction. After shipment to UCSF, the supernatant is centrifuged to isolate a largeaggregate surfactant fraction that is assayed as previously described 1 for both phospholipid andsurfactant prote<strong>in</strong> concentration under the direction of Dr. Philip Ballard. The supernatant fractionafter surfactant isolation is assayed for selected cytok<strong>in</strong>es us<strong>in</strong>g a multiplex assay. The TA cell pelletis used for isolation of DNA to develop a repository for genetic studies of gene variants. All trachealaspirate and DNA samples are labeled by study ID number and date/time, without other identifiers.Patient name, <strong>in</strong>itials, birth date and other potential identifiers are known only to <strong>in</strong>vestigators at eachstudy site and this <strong>in</strong>formation is not available to Dr. P. Ballard and other laboratory personnel. Thestudy samples are stored <strong>in</strong> a locked freezer and access to the Ballard laboratory requires a codedidentification card that is available only to approved laboratory personnel. Analyses of data fromstudy samples as related to cl<strong>in</strong>ical parameters is performed by personnel <strong>in</strong> the DCC. Should anyportion of samples rema<strong>in</strong>, we request that it be stored, labeled only by study number, <strong>in</strong> thelaboratory of Dr. P. Ballard for further test<strong>in</strong>g of substances identified as related to airway<strong>in</strong>flammation and the development of BPD (this is addressed specifically <strong>in</strong> the consent form andreflected <strong>in</strong> the CRF).10.2 Ur<strong>in</strong>e Sample Collection. Samples of ur<strong>in</strong>e will be collected from the <strong>in</strong>fants at regular <strong>in</strong>tervalsto 36 wk PMA and banked for analysis for markers of BPD and long term pulmonary morbidity(requires ancillary fund<strong>in</strong>g).11.0 ADVERSE EVENT AND PROTOCOL VIOLATION REPORTING11.1 Adverse Event Report<strong>in</strong>g. Adverse events will be recorded surround<strong>in</strong>g the study drugadm<strong>in</strong>istration, up until 7 d after dos<strong>in</strong>g is completed, at 36 wk PMA and through to discharge and asummary for each <strong>in</strong>fant will be reported by the sites to the CCC and DCC at 36 wk PMA anddischarge. See Table 11.111.1.a.Serious Adverse Events (SAE). The most important SAE is death occurr<strong>in</strong>g up to 7 d.after dos<strong>in</strong>g completed. This requires expedited report<strong>in</strong>g (see flow chart). In addition, severe,cardiopulmonary decompensation requir<strong>in</strong>g CPR with cardiac meds/compressions with<strong>in</strong> 4 h of dos<strong>in</strong>gor <strong>in</strong>crease <strong>in</strong> RSS of > 5 over basel<strong>in</strong>e and susta<strong>in</strong>ed > 24 h with<strong>in</strong> 4 h of a dos<strong>in</strong>g procedure, severe1/18/2011 21
pulmonary hemorrhage, pneumothorax requir<strong>in</strong>g a chest tube, or significant PIE with<strong>in</strong> 24 h of ados<strong>in</strong>g procedure, will be reported to the IRBs, CCC, DCC, NHLBI, the DSMB and the FDA by the<strong>in</strong>vestigators. Unexpected events thought to be related to the study drug will also be consideredSAEs. See flow chart for expected tim<strong>in</strong>g of report<strong>in</strong>g. A neonatologist <strong>in</strong>dependent of this study willserve as the data safety monitor<strong>in</strong>g officer, and will monitor all serious adverse events concurrently.Each death will be reported <strong>in</strong> a detailed letter to the FDA as well as to appropriate IRBs and theDSMB.11.1.b. Adverse Events. (see table 11.1)will primarily be i) related to study drug dos<strong>in</strong>g, ii) the<strong>in</strong>cidence of the known co-morbidities of prematurity <strong>in</strong> these critically ill <strong>in</strong>fants <strong>in</strong>clud<strong>in</strong>g sepsis, IVH,PVL, NEC, PDA, and ROP, iii) other relevant pulmonary or cardiovascular complications <strong>in</strong>clud<strong>in</strong>gpulmonary hypertension or airway abnormalities, iv) complications related to tracheal aspiratesampl<strong>in</strong>g, v) requirement for CPR <strong>in</strong>volv<strong>in</strong>g cardiac medications and chest compression, vi)unexpected events, and will be reported to the DSMB at regular <strong>in</strong>tervals as part of safety monitor<strong>in</strong>g.TABLE 11.1 PROPOSED SAE AND AE REPORTING(See Table 11.2 <strong>in</strong> MOP and Flow Chart for tim<strong>in</strong>g of report<strong>in</strong>g)SAEs (these are only with<strong>in</strong> study dos<strong>in</strong>g time plus 7 d)1. Death (only SAE requir<strong>in</strong>g expedited report<strong>in</strong>g)2. Beg<strong>in</strong>n<strong>in</strong>g with<strong>in</strong> 4 h of dos<strong>in</strong>g:a.Severe cardiopulmonary decompensation requir<strong>in</strong>g CPR with cardiac medication & chestcompressionsb. Increase <strong>in</strong> RSS >5 susta<strong>in</strong>ed for >24 hours3. With<strong>in</strong> 24 hrs of dos<strong>in</strong>g:a. Severe pulmonary hemorrhageb. Severe PIEc. Pneumothorax requir<strong>in</strong>g chest tube4. Unexpected and related to study drug adm<strong>in</strong>istrationAE’s1. Death >7 d after dos<strong>in</strong>g <strong>protocol</strong> (also fill out death form)2. Problems with obta<strong>in</strong><strong>in</strong>g tracheal aspirate samples3. With<strong>in</strong> 4 h of dos<strong>in</strong>g procedure (separate confidential form completed by dos<strong>in</strong>g team).a. Prolonged (>60 seconds)bradycardia/desaturationb. Endotracheal tube problems requir<strong>in</strong>g re<strong>in</strong>tubation4. CPR requir<strong>in</strong>g cardiac meds and compressions5. Hypotension requir<strong>in</strong>g vasopressor support w/ dopam<strong>in</strong>e > 20 mcg/kg/m<strong>in</strong> or 2 pressor agents for >24 h6. Co-Morbidities occurr<strong>in</strong>g after enrollment <strong>in</strong> study (details are described <strong>in</strong> CRF and MOP)a. Neurologic (IVH, PVL, hydrocephalus)b. GI (NEC, perforation, surgery)c. Pulmonary (PIE, pulmonary hemorrhage, pneumothorax, tracheomalacia, stenosis)d. Cardiovascular (PDA -with or without surgery, pulmonary hypertension)e. Sepsis (bacterial, fungal, viral)7. Unexpected adverse events (medication errors, catheter complications etc)NOT TO BE CONSIDERED AEs – common problems encountered <strong>in</strong> the cl<strong>in</strong>ical care of these <strong>in</strong>fantssuch as feed<strong>in</strong>g <strong>in</strong>tolerance, or electrolyte imbalance will not be considered AEs (see MOP)1/18/2011 22
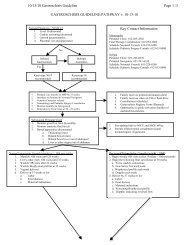
TABLE 11.3 SERIOUS ADVERSE EVENT/ADVERSE EVENT FLOWCHARTSAE’s <strong>in</strong>clude the follow<strong>in</strong>g events:a) death if it occurs between enrollment and 1 week (7 days) after dos<strong>in</strong>g completed:b) severe respiratory decompensation requir<strong>in</strong>g CPR with chest compressions and cardiac meds with<strong>in</strong> 4 hours of dos<strong>in</strong>gc) Increase <strong>in</strong> RSS >5 from basel<strong>in</strong>e with<strong>in</strong> 4 hours of dos<strong>in</strong>g susta<strong>in</strong>ed for >24 hoursd) severe pulmonary hemorrhage, severe PIE, or pneumothorax with<strong>in</strong> 24 hours of dos<strong>in</strong>ge) unexpected and related to study drug adm<strong>in</strong>istrationEVENT OCCURRED BETWEEN ENROLLMENT AND 7 DAYS AFTER DOSING COMPLETED:Adverse Event Occurs:Is AEDeath?(SAE)NOYESSites Notify CCCwith<strong>in</strong> 72 h ofoccurrenceCCC notifies DCCCCC notifies FDAWith<strong>in</strong> 7 daysDCC notifiesNHLBI,and DSMBwith<strong>in</strong> 3 days ofnotificationWith<strong>in</strong> 72 hrs:1. Fax SAE CRF 21 andDeath Form CRF 17 toDCC2. Fax Death Summary toCCC3. Sites notify local IRB(depend<strong>in</strong>g on site IRB<strong>protocol</strong>)Is AE an SAE dueto expected,serious, lifethreaten<strong>in</strong>gevents <strong>in</strong> b,cor,dabove?YESSites Notify CCCWith<strong>in</strong> 7 dCCC notifies DCCWith<strong>in</strong> 15 d:1. Fax SAE CRF 21 to DCC2. Sites notify IRB(depend<strong>in</strong>g on site IRB<strong>protocol</strong>)NOCCC notifies FDADCC notifies NHLBI, andDSMB with<strong>in</strong> 15 dIs AEunexpected andrelated to thestudy drugadm<strong>in</strong>istration?YESDCC DSMBreportQ6moNOIs AE an expected,prematurity relatedevent?OrUnexpected but notrelated to study drugYESWith<strong>in</strong> 2 wk of end of dos<strong>in</strong>g period faxAE Summary CRF 13A to DCCDCC DSMBReport Q ~6 Mo1/18/2011 23
EVENT OCCURRED > 7 DAYS AFTER DOSING COMPLETED:Adverse Event occursIs AEDeath?YESNotify CCC,&DCC,Complete DeathForm CRF 17 send toDCC <strong>in</strong> 2 wkNOIs AEunexpectedOr otherYESComplete AE SummaryCRF 13B at D/CNOIs AE anexpected,prematurityrelatedevent?YESWith<strong>in</strong> 1-2 wk of discharge period sites fax AESummary CRF 13B to DCC;DCC DSMBReport ~ Q 6 mo11.2 Protocol Violation/Deviation Report<strong>in</strong>g. Violations of the study drug adm<strong>in</strong>istration <strong>protocol</strong>require completion of a Protocol Violation Form and notification to the CCC and DCC with<strong>in</strong> 3work<strong>in</strong>g d.11.2.a Major Protocol Violations Include:- Enrollment <strong>in</strong> light of exclusion criteria- Consent obta<strong>in</strong>ed not <strong>in</strong> accordance with IRB guidel<strong>in</strong>es- Unbl<strong>in</strong>d<strong>in</strong>g of study personnel- Study drug adm<strong>in</strong>istration or dos<strong>in</strong>g error- Withdrawal from study <strong>protocol</strong> for any reason- Failure to perform oxygen/flow reduction test as <strong>in</strong>dicated- Inhaled NO not given- Open label surfactant adm<strong>in</strong>istered11.2.b Protocol Deviations <strong>in</strong>clude:- iNO not given accord<strong>in</strong>g to <strong>protocol</strong>- deviation from cl<strong>in</strong>ical guidel<strong>in</strong>es1/18/2011 24
12.0 STATISTICAL CONSIDERATIONS12.1 Sample Size and Power.12.1.a. Assumptions for calculation of sample size. Our calculations are based on acomparison of the proportion of <strong>in</strong>fants who achieve the primary endpo<strong>in</strong>t (BPD-free survival at 36 wk)<strong>in</strong> the two treatment groups. The detailed analysis methods are described <strong>in</strong> more detail below. Insummary, we use a significance level of 0.0488, reflect<strong>in</strong>g a s<strong>in</strong>gle <strong>in</strong>terim analysis. In analyses of36-wk BPD-free survival among <strong>in</strong>fants 7-14 d old at study entry <strong>in</strong> the NO CLD trial, the <strong>in</strong>flation ofthe variance of the treatment effect estimate <strong>in</strong> GEE analysis, relative to an analysis assum<strong>in</strong>g<strong>in</strong>dependence, is approximately 1.05. This value is used <strong>in</strong> the sample size calculation.12.1.b. Placebo/ iNO-only event rate. The primary endpo<strong>in</strong>t for the study is BPD-free survival at36 wk. This study will be limited to <strong>in</strong>fants between 7 and 14 d, who comprised about half the NO CLDsample, and benefited more than older <strong>in</strong>fants. Specifically, the BPD-free survival rate <strong>in</strong> this groupwas 49.1% <strong>in</strong> the iNO group 2, 3 , and 44% <strong>in</strong> the subgroup of these <strong>in</strong>fants who were <strong>in</strong>tubated atenrollment (<strong>in</strong>fants could be enrolled <strong>in</strong> NO CLD who were on NCPAP). On this basis we haveassumed that the 36-wk BPD-free survival rate will be 44% <strong>in</strong> the iNO-only group <strong>in</strong> the proposedstudy. The impact of alternative assumptions are shown <strong>in</strong> section 12.1.e below.12.1.c. Treatment effect size. In design<strong>in</strong>g the NO CLD trial 2, 3 , we calculated the sample sizeto ensure power to detect a 12.5% absolute <strong>in</strong>crease <strong>in</strong> 36-wk BPD-free survival. The 12.5% absoluteimprovement was felt to be cl<strong>in</strong>ically important at that time and we believe is still a relevant magnitudeof effect. In design<strong>in</strong>g the current trial, we have <strong>in</strong>creased the absolute improvement slightly to 12.9%<strong>in</strong> order to accommodate our approved samples size of 524. Given a 44% disease-free survivalproportion <strong>in</strong> the iNO-only (surfactant placebo) group, this would correspond to a 36-wk BPD-survivalrate of 56.9% <strong>in</strong> the NO+surfactant group. For comparison purposes, the treatment effect observed <strong>in</strong>the NO CLD study <strong>in</strong> the 7 to 14 d subgroup was much larger: disease-free survival to 36 wk was25% <strong>in</strong> the placebo group compared to 44% <strong>in</strong> the NO-treated group (an absolute difference of 19%).The impact of alternative treatment effect assumptions are shown <strong>in</strong> section 12.1.e below.12.1.d. Sample size results. Us<strong>in</strong>g these parameter estimates and standard methods for b<strong>in</strong>aryoutcomes, we calculate that a sample of 524 will provide 80% power <strong>in</strong> 2-sided tests to detect theproposed <strong>in</strong>crease <strong>in</strong> 36-week BPD-free survival from 44% to 56.9%.12.1.e Power and sample size under alternative <strong>in</strong>cidence and effect size assumptions. The twotables below show the power and sample size under alternative assumptions about the <strong>in</strong>cidence rate<strong>in</strong> the placebo arm and the (absolute) <strong>in</strong>crease <strong>in</strong> the surfactant arm.Table: Power (n=524) for alternative <strong>in</strong>cidence and effect sizesIncrease <strong>in</strong> outcome <strong>in</strong> surfactant arm6% 9% 12% 13%* 14% 15%40% 24.0 48.9 74.2 80.1 86.5 90.9Incidence <strong>in</strong> placebo 44% 23.7 48.4 73.9 80 86.4 90.848% 23.6 48.5 74.2 80.3 86.8 91.1Sample size (power=.8) for alternative <strong>in</strong>cidence and effectsizesIncrease <strong>in</strong> outcome <strong>in</strong> surfactant arm6% 9% 12% 13%* 14% 15%40% 2320 1050 599 521 443 387Incidence <strong>in</strong> placebo 44% 2357 1061 603 524 445 38848% 2364 1060 599 520 441 384*Actual percentage 12.9%, rounded to 13%12.2.General Statistical analysis considerations. An <strong>in</strong>tention-to-treat (ITT) approach 56 will be used,with all randomized <strong>in</strong>fants to be <strong>in</strong>cluded <strong>in</strong> the analysis. Tests of the primary hypothesis of the effect1/18/2011 25
on treatment on BPD-free survival at 36 wk will use a two-sided significance level of 0.0488, to reflecta s<strong>in</strong>gle <strong>in</strong>terim efficacy analysis us<strong>in</strong>g O’Brien-Flem<strong>in</strong>g boundaries. All secondary hypotheses will betested at the nom<strong>in</strong>al 5% 2-sided significance level, with no formal adjustment for multiplecomparisons. Our <strong>in</strong>ference will be focused on the primary outcome. In the analysis of secondaryoutcomes and <strong>in</strong> other secondary analyses, we recognize that this might <strong>in</strong>crease type-I error rates,due to multiple test<strong>in</strong>g. To alleviate that concern, we will clearly dist<strong>in</strong>guish primary from secondaryendpo<strong>in</strong>ts as well as exploratory analyses, look for consistency among multiple endpo<strong>in</strong>ts, and becautious <strong>in</strong> our <strong>in</strong>terpretation of marg<strong>in</strong>ally significant f<strong>in</strong>d<strong>in</strong>gs, particularly unexpected results notmotivated by a priori hypotheses.12.2.a. Cluster<strong>in</strong>g of outcomes with<strong>in</strong> multiple births. Outcomes <strong>in</strong> this study will potentially beclustered with<strong>in</strong> multiple-birth sibships. To address this, we will use generalized estimat<strong>in</strong>g equations(GEE) models with robust standard 57 for cont<strong>in</strong>uous and b<strong>in</strong>ary outcomes, treat<strong>in</strong>g gestational agestratum as a fixed effect. GEE models are implemented <strong>in</strong> Proc Genmod <strong>in</strong> SAS.12.2.b. Miss<strong>in</strong>g data. The extent of miss<strong>in</strong>g data will be reported <strong>in</strong> all statistical analyses. Forthe primary outcome, the overall rate is expected to be low, s<strong>in</strong>ce most measurements are to beobta<strong>in</strong>ed while the <strong>in</strong>fants are hospitalized, none are <strong>in</strong>vasive, and almost all are part of regularcl<strong>in</strong>ical care. Therefore, no specific miss<strong>in</strong>g data imputation methods are considered for the basel<strong>in</strong>edata and short-term outcomes. If there are cases with miss<strong>in</strong>g data for the primary outcome, they willbe considered to have BPD. Covariate adjustment will be used <strong>in</strong> the analysis of longer-termendpo<strong>in</strong>ts to account for between-group differences potentially aris<strong>in</strong>g from mortality and loss tofollow-up (see below). Additional sensitivity analyses will be undertaken us<strong>in</strong>g multiple imputation ofthe miss<strong>in</strong>g outcomes among surviv<strong>in</strong>g <strong>in</strong>fants 58 .12,2,c. Analyses restricted to surviv<strong>in</strong>g <strong>in</strong>fants rema<strong>in</strong><strong>in</strong>g <strong>in</strong> follow-up. Some secondaryoutcomes, <strong>in</strong> particular pulmonary status at 1 yr and neurodevelopmental impairment at 2 yr correctedage, will be analyzed only among surviv<strong>in</strong>g <strong>in</strong>fants who rema<strong>in</strong> <strong>in</strong> follow up. To deal with potentialconfound<strong>in</strong>g of treatment effects by differences among the <strong>in</strong>fants available for re-assessment, we willuse regression models to adjust for all important basel<strong>in</strong>e predictors of both mortality and the outcomeof direct <strong>in</strong>terest. Beg<strong>in</strong>n<strong>in</strong>g with prognostic covariates at least weakly associated with death or loss tofollow-up, we will use backward deletion with a liberal retention criterion to select covariates for thesemodels.12.3 Statistical Analysis Plan: Primary Outcome. The primary analyses will use a GEE logistic modelto assess the effects of treatment on the proportion of <strong>in</strong>fants alive and free of BPD at 36 wk. Thismodel will <strong>in</strong>clude randomization stratum as a covariate. We expect that BPD-free survival status at36 weeks will be observed for all <strong>in</strong>fants.In this analysis, BPD-free survival, is treated as a dichotomous (yes/no) variable. It cannot betreated as a conventional survival outcome, because at randomization, all <strong>in</strong>fants are alive but meetthe criteria for possible BPD. Over the 36-wk follow-up, two events might occur: resolution of lungdisease or death. The complication is that at 36 wk, treatment failure is def<strong>in</strong>ed by censor<strong>in</strong>g of time toresolution of BPD, or occurrence of death, while treatment success is def<strong>in</strong>ed by resolution of BPDsigns and symptoms, plus censor<strong>in</strong>g of time to death. This is <strong>in</strong> contrast to usual cl<strong>in</strong>ical eventendpo<strong>in</strong>t trials (eg., cancer trials) assess<strong>in</strong>g recurrence-free survival, for example, where failure isdef<strong>in</strong>ed by tumor recurrence or death, and success is def<strong>in</strong>ed by censor<strong>in</strong>g of both failure timeoutcomes. Because follow-up at 36 wk is expected to be complete, we resolved this difficulty bypropos<strong>in</strong>g use of a dichotomous outcome with GEE logistic model for the b<strong>in</strong>ary outcome def<strong>in</strong>ed asbe<strong>in</strong>g alive and free of BPD at 36 wk.12.4 Statistical Analysis Plan: Secondary Analyses Of Primary Outcome12.4.a. Check<strong>in</strong>g balance by treatment on basel<strong>in</strong>e covariates. We expect randomization toresult <strong>in</strong> well-balanced basel<strong>in</strong>e characteristics, particularly for gestational age and cl<strong>in</strong>ic, factors onwhich the randomization will be stratified. We will assess balance between treatment groups, <strong>in</strong> termsof age at randomization, gestational age, gender, race, IVH, severity score, surfactant status, previousextubation, and sepsis. On the basis of this assessment, we will perform secondary analyses oftreatment effects adjust<strong>in</strong>g for any basel<strong>in</strong>e prognostic characteristics sufficiently imbalanced toconfound the treatment effect estimates.1/18/2011 26
12.4.b. Subgroup analyses. We will perform pre-specified subgroup analyses to exam<strong>in</strong>edifferences <strong>in</strong> the effect of treatment across important subgroups as def<strong>in</strong>ed by gestational age (
difference between treatment groups significant at a p< 0.1, the rema<strong>in</strong><strong>in</strong>g 400-424 samples will beanalyzed. We consider this a reasonable tradeoff between type-I and type-II error <strong>in</strong> the <strong>in</strong>itialscreen<strong>in</strong>g. We do not plan to adjust the significance levels for results us<strong>in</strong>g all samples, but willcautiously <strong>in</strong>terpret them as hypothesis generat<strong>in</strong>g and requir<strong>in</strong>g confirmation.Because a large number of exploratory analyses will be performed, with the potential to <strong>in</strong>flate thetype-I error rate, the results will be <strong>in</strong>terpreted cautiously, and used to help <strong>in</strong>terpret results for theprimary and secondary outcomes or as hypothesis-generat<strong>in</strong>g.13.0 DATA COLLECTION AND MANAGEMENT13.1 Data Collection. All data will be collected on mach<strong>in</strong>e-readable case report forms (CRFs) thatare developed specifically for the study. These forms will <strong>in</strong>clude screen<strong>in</strong>g, enrollment and follow-updata for <strong>in</strong>fants.The <strong>in</strong>fant’s parent or legal guardian must read, understand, and sign an IRB approved <strong>in</strong>formedconsent form. Additionally, it should be recorded <strong>in</strong> the <strong>in</strong>fant’s medical record that the <strong>in</strong>fant is<strong>in</strong>volved <strong>in</strong> this study. The Investigator must reta<strong>in</strong> the signed consent form <strong>in</strong> a secured location.Investigators must provide all <strong>in</strong>formation required by the <strong>protocol</strong> on the CRFs provided for thestudy (see Manual of Operations).13.2 Data Management. The UCSF Data Coord<strong>in</strong>at<strong>in</strong>g Center (DCC) data system comb<strong>in</strong>esdecentralized data submission, centralized and remote data edit<strong>in</strong>g, and a centralized databasestructure designed to collect, transfer, and store data for large-scale multi-center cl<strong>in</strong>ical studies.Cl<strong>in</strong>ical sites submit data to the DCC on mach<strong>in</strong>e-readable, validated case report forms (CRFs) viasecure fax. After the data have been electronically received by the DCC, they are assessed for qualityvia automated and manual processes and then written to the study database. Queries (datadiscrepancies) are generated (hourly dur<strong>in</strong>g bus<strong>in</strong>ess hours) to identify potential errors <strong>in</strong> the studydata and are immediately accessible via a secure study web site so that the CCC project director (PD)can consult with the onsite study coord<strong>in</strong>ator to resolve them <strong>in</strong> a timely manner. Orig<strong>in</strong>al sourcedocuments are kept by the site <strong>in</strong> a secure location for their records.The study coord<strong>in</strong>ator at each site (or designate) will review all CRFs for completeness, accuracyand consistency prior to fax<strong>in</strong>g <strong>in</strong>to the data system. Should any questions arise dur<strong>in</strong>g this review, allqueries will be referred back to the site <strong>in</strong>vestigator for resolution prior to fax<strong>in</strong>g the forms to the DCC.After forms are submitted to the DCC, they are subject to image verification by a DCC tra<strong>in</strong>ed dataeditor and then exported to a SQL database where metadata tables authenticate the data as valid andpre-programmed logic checks look for discrepant or miss<strong>in</strong>g data. Data queries are posted to a securewebsite and addressed by the CCC PD <strong>in</strong> consultation with the onsite study coord<strong>in</strong>ator. Queryresolution that requires a change <strong>in</strong> how the data form is completed are recorded on the paper form,dated and <strong>in</strong>itialed at the cl<strong>in</strong>ical site. The CCC PD is responsible for enter<strong>in</strong>g data changes us<strong>in</strong>g thedata management tools on the secure, password-protected study website. An audit table generateddur<strong>in</strong>g the edit<strong>in</strong>g process conta<strong>in</strong>s a complete record of the changes and automatically generates anaudit trail.Components of the web site dedicated to data management operations <strong>in</strong>clude the follow<strong>in</strong>gfeatures:- data query and edit tables- miss<strong>in</strong>g and rejected forms list<strong>in</strong>g- data <strong>in</strong>ventory- audit trail13.3 Data Audit. After all patients’ data have been submitted and data queries addressed, thedatabase will be considered ready to lock. At this time, a random 10% sample of patients will beaudited (all data po<strong>in</strong>ts) for accuracy between the source paper CRF at the cl<strong>in</strong>ical site and the studydatabase.13.4 Data Security and Confidentiality. The DCC employs procedural and technical controls to ensurethe security, <strong>in</strong>tegrity and confidentiality of subject data that are <strong>in</strong> compliance with established1/18/2011 28
standards for Information Technology Security. Multiple levels of data security are <strong>in</strong> place designedto prevent unauthorized access and limit authorized access to the computer systems and preventdata corruption and loss.At the procedural level, all DCC personnel sign a confidentiality agreement and undergo securityawareness tra<strong>in</strong><strong>in</strong>g for HIPAA and sensitive data handl<strong>in</strong>g. Remote users also receive tra<strong>in</strong><strong>in</strong>g prior toga<strong>in</strong><strong>in</strong>g access to the data system. Access to study data with<strong>in</strong> the system is granted on as asneededbasis and further restricted by def<strong>in</strong>ed user roles. User authentication is by unique usernameand password. User accounts are configured to lock out a user after three failed log-<strong>in</strong> attempts andto log-off after 30 m<strong>in</strong>utes of <strong>in</strong>activity. Study website and database access requires a networkdoma<strong>in</strong> account with appropriate account-specific permissions on the database. All requests for newaccounts and access to the database must be documented by a System Access Request Form andsigned by the DCC PD. Subject files conta<strong>in</strong><strong>in</strong>g the orig<strong>in</strong>al CRFs are ma<strong>in</strong>ta<strong>in</strong>ed at the cl<strong>in</strong>ical sites.Website communications are encrypted at the 128-bit level us<strong>in</strong>g an SSL certificate issued by Verisign(Verisign, Inc, Mounta<strong>in</strong> View, CA).Computer programs are fully documented tested and subject to change controls <strong>in</strong> concurrencewith FDA-mandated requirements for pharmaceutical studies. Audit trails are automatically generatedfor both study data and for meta-data (i.e., forms, query logic, data dictionary, and log-on table)housed <strong>in</strong> SQL databases, which show who made a change, date and time a change was made,description of the data or meta-data item affected, the old and new value, and the reason for thechange.The DCC network is privately ma<strong>in</strong>ta<strong>in</strong>ed, hardware fire-walled and none of the workstations ordatabase servers can be directly addressed from outside the Local Area Network. All study data arestored on SQL servers at the DCC at 185 Berry St., San Francisco. All servers are housed <strong>in</strong> a new(2005) state-of the-art secure server room. Access to the server room is via a limited access suiteoccupied by the Information Technology (IT) staff. Both the suite and server room doors are fittedwith an Access Control System. Only critical IT staff members are allowed to enter the room. Allothers who enter the server room (e.g. air condition<strong>in</strong>g repairman) must be accompanied by amember of the IT staff and their visit is logged. Servers are protected from viruses by Network<strong>Associates</strong> Netshield 4.x, Groupshield, and VirusScan Enterprise 7.x (McAfee, Santa Clara, CA).This software automatically checks for virus signature file updates from Network <strong>Associates</strong>’ FTP andHTTP sites once an hour. Anti-virus software is monitored and IT personnel are notified <strong>in</strong> the eventthat the software stops function<strong>in</strong>g on a particular server.Each server is backed-up nightly to disk and mirrored to a “failover” site at the co-location facilityat 650 Townsend St., San Francisco. These two sites have copies of the study database and allassociated systems required to carry on the study <strong>in</strong> the event of a disaster <strong>in</strong> one of the locations. Inaddition, back up copies of the entire enterprise (databases, user workstations, file servers, etc) arearchived <strong>in</strong> Sacramento, California by Recall, Inc. This will protect the study data <strong>in</strong> case of a naturaldisaster affect<strong>in</strong>g the San Francisco Bay Area.The disclosure of <strong>in</strong>dividual health <strong>in</strong>formation will comply with local, state, and federal laws andregulations (<strong>in</strong>clud<strong>in</strong>g the Privacy Rule under the Health Insurance Portability and Accountability Act[HIPAA] of 1996) relat<strong>in</strong>g to the privacy, security and confidentiality of health <strong>in</strong>formation collected forresearch purposes. Subject confidentiality will be protected thorough a multi-tiered approach.13.4.a. Consent. Only subjects with a signed IRB-approved consent form at the cl<strong>in</strong>ical center willhave their data <strong>in</strong>cluded <strong>in</strong> a DCC dataset.13.4.b Subject identifiers. Subject data submitted to the DCC are identified by a study ID number(screen<strong>in</strong>g ID), random acrostic secondary ID, and randomization number. Only the cl<strong>in</strong>ical center willhave the key that maps the ID number, <strong>in</strong>fant <strong>in</strong>itials and randomization number to the subject’s nameand contact <strong>in</strong>formation at their site. At the cl<strong>in</strong>ical sites, all subject data are ma<strong>in</strong>ta<strong>in</strong>ed <strong>in</strong> locked filecab<strong>in</strong>et secure locations with limited access by researchers and staff. Laboratory samples will beidentified by a study ID number, secondary ID and randomization number. Tracheal aspirate sampleswill be stored <strong>in</strong> the laboratory of Philip Ballard MD at UCSF. A list of each sites’ subjects will be keptseparately <strong>in</strong> password protected or locked files with<strong>in</strong> that site <strong>in</strong>vestigator’s office.13.4.c. Data submission. Data are sent from the cl<strong>in</strong>ical sites to the DCC by secure fax to theDCC network.1/18/2011 29
14.0 DOCUMENTATION AND RECORD RETENTION14.1 Documentation. Each site must provide the CCC project director or designate with the follow<strong>in</strong>gdocuments prior to study <strong>in</strong>itiation. A copy of these documents must be ma<strong>in</strong>ta<strong>in</strong>ed <strong>in</strong> the<strong>in</strong>vestigator’s study files.- IRB approved <strong>in</strong>formed consent form- All IRB approvals and correspondence (<strong>in</strong>clud<strong>in</strong>g approved revisions, <strong>protocol</strong>, advertisements,etc.)- Copies of all correspondence perta<strong>in</strong><strong>in</strong>g to the study (exclud<strong>in</strong>g any budgetary matters)- Copies of all serious adverse events submitted to the IRB- Copy of all safety reports.14.2 Record Retention. The cl<strong>in</strong>ical site is responsible for ma<strong>in</strong>ta<strong>in</strong><strong>in</strong>g all records (i.e., case reportforms, orig<strong>in</strong>al data, screen<strong>in</strong>g logs, signed <strong>in</strong>formed consent forms, correspondence, etc.) untilnotified, <strong>in</strong> writ<strong>in</strong>g, by CCC, that these records are no longer needed. The Investigator must notifyCCC project director if the site or records are relocated, if the <strong>in</strong>vestigator leaves the <strong>in</strong>stitution, etc.and a new address for the records must be provided.15.0 INTERIM SUMMARIES AND ANALYSES FOR THE DSMB15.1 Interim Summaries. The DSMB will be provided with <strong>in</strong>terim summaries of recruitment anddemographics, and periodically with summaries of safety. Initially, these reports will be created semiannuallyand the frequency will <strong>in</strong>crease or decrease at the discretion of the DSMB. They willsummarize data accrual and quality, show whether recruitment is proceed<strong>in</strong>g as scheduled, andwhether all sites are recruit<strong>in</strong>g approximately as expected. The reports will <strong>in</strong>clude demographics andsome basel<strong>in</strong>e characteristics, such as distribution among the two gestational age groups, and birthweight. Safety summaries will consist of adverse events tallies <strong>in</strong>clud<strong>in</strong>g response to study drugdos<strong>in</strong>g. The safety summaries will be by treatment, labeled as A and B. We will provide a summary<strong>in</strong>dividual report for each death, and for any unexpected events. We will summarize study drugprocedure <strong>in</strong>terruptions or errors, prematurity-related adverse events while on treatment + 7 days, andunexpected events while on treatment. We will also summarize all other adverse events on thosepatients followed to outcome and discharge. Percentages and differences by treatment will beprovided with confidence <strong>in</strong>tervals. No formal statistical <strong>in</strong>ference will be performed. The summarieswill be overall, by gestational age group, sex, race, severity and relationship to treatment as assessedby the site <strong>in</strong>vestigator.The CCC PI will be see<strong>in</strong>g the overall number of adverse events (not by treatment) <strong>in</strong> the opensession reports and <strong>in</strong> on-go<strong>in</strong>g monitor<strong>in</strong>g activities between DSMB meet<strong>in</strong>gs. If it is noticed that anadverse event is occurr<strong>in</strong>g at a higher frequency than expected, and this was not apparent at aprevious summary for the DSMB, the DCC will forward a summary of the event between reports to thechair of the DSMB, who will determ<strong>in</strong>e whether to convene the DSMB and what further <strong>in</strong>formation isneeded.15.2 Interim Analysis. Two formal efficacy <strong>in</strong>terim analyses will be performed. The first will beperformed when 25% of the data are complete to 36 weeks (131 <strong>in</strong>fants) and the second when 50%of the data have been collected (262 <strong>in</strong>fants). The data for the second <strong>in</strong>terim analysis is expected tobe available for analysis approximate 26 months after active enrollment start. The <strong>in</strong>terim analysis willaddress efficacy outcomes of the study and will report p-values. Formal stopp<strong>in</strong>g rules for the efficacyoutcomes have been established as a critical value of 4.33 and 2.96 at the first and second <strong>in</strong>terimanalyses respectively. In order to ma<strong>in</strong>ta<strong>in</strong> the overall significance level at 0.05, a small adjustmentwill be made <strong>in</strong> the f<strong>in</strong>al significance value from 1.96 to 1.9686 to account for the <strong>in</strong>terim analyses. Inaddition, a futility analysis will be carried out by the DCC and presented to the DSMB with each<strong>in</strong>terim analyses. Additional details of the <strong>in</strong>terim analysis plan and stopp<strong>in</strong>g rules are provided <strong>in</strong> theData and Safety Monitor<strong>in</strong>g Board Charter.1/18/2011 30
16.0 PROTOCOL AMENDMENTSAny changes to the <strong>protocol</strong> require a written <strong>protocol</strong> amendment that must be approved by Steer<strong>in</strong>gCommittee and the DSMB prior to implementation. Amendments that affect patient eligibility, study<strong>protocol</strong>, or consent changes require additional approval by the IRB at each site. Theseamendments, should they be required, will become a part of the <strong>protocol</strong> and ma<strong>in</strong>ta<strong>in</strong>ed by theInvestigator as part of the study documentation.Amendments affect<strong>in</strong>g only adm<strong>in</strong>istrative aspects of the study do not require formal IRB approval,however the IRB at each of the sites must be <strong>in</strong>formed of such adm<strong>in</strong>istrative changes.16.1 Other Changes In Study Conduct. Changes <strong>in</strong> the study conduct are not permitted. Anyunforeseen changes must be recorded <strong>in</strong> the cl<strong>in</strong>ical study report.17.0 STUDY ORGANIZATION AND ADMINISTRATIONThe PI (RAB) will chair an executive committee consist<strong>in</strong>g of Drs. P. Ballard, J. Merrill, R. Keller, D.Black, the NHLBI Project Scientist Carol Blaisdell and the Project Director, (N Newton) that will meetat least monthly to assure smooth operation of all aspects of the study. The PI will also chair ameet<strong>in</strong>g <strong>in</strong>itially monthly and at least quarterly (conference call) of the cl<strong>in</strong>ical steer<strong>in</strong>g committee,which <strong>in</strong>cludes members of the executive committee, appropriate staff from NHLBI as described <strong>in</strong> theUO1 terms and agreement document, as well as Drs. Truog, Ste<strong>in</strong>horn, Mammel, Durand, andEichenwald and the Research Coord<strong>in</strong>ator(J Assel<strong>in</strong>) to guide cl<strong>in</strong>ical aspects of the trial. The studyCCC PI will be available on a daily basis for communication with the Associate Directors (JM and RK),the Project Director and the Research Coord<strong>in</strong>ator as was the case <strong>in</strong> the NO CLD trial. This CCCcore group will meet (teleconference as necessary) along with the director of the laboratorycomponent (P Ballard) at least weekly to discuss trial operations and logistical issues as well as todevelop recommendations for the steer<strong>in</strong>g and/or executive committees.18.0 POTENTIAL RISKS, BENEFITS AND ALTERNATIVES18.1 Potential Risks. The ma<strong>in</strong> potential risks for this study are the expected risks of surfactantadm<strong>in</strong>istration. The most common adverse reactions associated with Infasurf dos<strong>in</strong>g are cyanosis,airway obstruction, bradycardia, reflux of the surfactant <strong>in</strong> the endotracheal tube, requirement formanual ventilation and re<strong>in</strong>tubation. (see MOP for specific def<strong>in</strong>itions) These events are usuallytransient and not associated with serious complications. We will m<strong>in</strong>imize this risk by hav<strong>in</strong>grespiratory therapists tra<strong>in</strong>ed <strong>in</strong> surfactant adm<strong>in</strong>istration present dur<strong>in</strong>g study drug <strong>in</strong>stillation,standardized <strong>in</strong>stillation and ventilatory management <strong>protocol</strong>s across all study sites, as well asphysicians present with<strong>in</strong> the nursery dur<strong>in</strong>g drug adm<strong>in</strong>istration, should the <strong>in</strong>fant need to bere<strong>in</strong>tubated. We feel that the risks of surfactant adm<strong>in</strong>istration to neonates beyond the first week oflife are equivalent to those of neonates given rout<strong>in</strong>e surfactant <strong>in</strong> the first 72 h of life, as evidenced byour prior prophylactic pilot trials’ safety data. In addition, the studies evaluat<strong>in</strong>g surfactantreplacement <strong>in</strong> neonates with early chronic lung disease 19-21 reported improvement <strong>in</strong> oxygenation,without untoward effects.All the <strong>in</strong>fants <strong>in</strong> this study will be receiv<strong>in</strong>g iNO therapy modeled after the regimen used <strong>in</strong> the NOCLD trial. An Information Sheet about iNO <strong>in</strong> the Preterm Infant will be provided as part of the consentprocedure. If, for any reason, the cl<strong>in</strong>ical team feels that the <strong>in</strong>fant does not tolerate iNO, the gas willbe discont<strong>in</strong>ued. The <strong>in</strong>fant will rema<strong>in</strong> <strong>in</strong> the study and all other study procedures will cont<strong>in</strong>ue.18.2 Potential Benefits. Those <strong>in</strong> the surfactant treatment arm may experience improvement <strong>in</strong>oxygenation and ventilation allow<strong>in</strong>g a reduction <strong>in</strong> airway pressures and <strong>in</strong>spired oxygenconcentration after surfactant therapy. There may be no direct benefit from surfactant treatment. Therisk/benefit ratio is favorable.1/18/2011 31
9. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of theNational Institutes of Health consensus def<strong>in</strong>ition of bronchopulmonary dysplasia. Pediatrics.2005;116(6):1353-1360.10. Whitsett JA, Weaver TE. Hydrophobic surfactant prote<strong>in</strong>s <strong>in</strong> lung function and disease. N EnglJ Med. 2002;347(26):2141-2148.11. Ballard PL, Ballard RA, Merrill JD, Cnaan A, Haque A, God<strong>in</strong>ez M, God<strong>in</strong>ez R. Composition ofpulmonary surfactant <strong>in</strong> premature <strong>in</strong>fants. Pediatr Res. 2003;53(4):353A.12. Merrill JD, Ballard RA. Pulmonary surfactant for neonatal respiratory disorders. Curr Op<strong>in</strong>Pediatr. 2003;15(2):149-154.13. Soll RF, Blanco R. Natural surfactant extract versus synthetic surfactant for neonatalrespiratory distress syndrome. Cochrane Database Syst Rev. 2001;CD000144(2).14. Gunther A, Schmidt R, Harodt J, Schmehl T, Walmrath D, Ruppert C, et al. Bronchoscopicadm<strong>in</strong>istration of bov<strong>in</strong>e natural surfactant <strong>in</strong> ARDS and septic shock: impact on biophysicaland biochemical surfactant prote<strong>in</strong>s. Eur Respir J. 2002;19:797-804.15. Mander A, Langton-Hewer S, Bernhard W, Warner JO, Postle AD. Altered phospholipidcomposition and aggregate structure of lung surfactant is associated with impaired lungfunction <strong>in</strong> young children with respiratory <strong>in</strong>fections. Am J Respir Cell Mol Biol. 2002;27:714-721.16. Skelton R, Holland P, Darowski M, Chetcuti PA, Morgan LW, Harwood JL. Abnormalsurfactant composition and activity <strong>in</strong> severe bronchiolitis. Acta Paediatr. 1999;88(9):942-946.17. Gunther A, Schmidt R, Nix F, Abut-Perez M, Guth C, Rosseau S, et al. Surfactantabnormalities <strong>in</strong> idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and saccoidosis.Eur Respir J. 1999;14:565–573.18. Schmidt R, Markart P, Ruppert C, Temmesfeld B, Nass R, Lohmeyer J, et al. Pulmonarysurfactant <strong>in</strong> patients with Pneumocystis pneumonia and acquired immunodeficiencysyndrome. Crit Care Med. 2006;34(9):2379-2376.19. Pandit PB, Dunn MS, Kelly EN, Perlman M. Surfactant replacement <strong>in</strong> neonates with earlychronic lung disease. Pediatrics. 1995;95(6):851-854.20. Biss<strong>in</strong>ger R, Carlson C, Hulsey T, Eicher D. Secondary surfactant deficiency <strong>in</strong> neonates. JPer<strong>in</strong>atol. 2004;24(10):663-666.21. Katz LA, Kle<strong>in</strong> JM. Repeat surfactant therapy for postsurfactant slump. J Per<strong>in</strong>atol.2006;26(7):414-422.22. Shaul PW. Nitric oxide <strong>in</strong> the develop<strong>in</strong>g lung. Adv Pediatr. 1995;42:367-414.23. Shaul PW, Afshar S, Gibson LL, Sherman TS, Kerecman JD, Grubb PH, et al. Developmentalchanges <strong>in</strong> nitric oxide synthase isoform expression and nitric oxide production <strong>in</strong> fetal baboonlung. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1192-L1199.24. Bland RD, Albert<strong>in</strong>e KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lungstructure and function <strong>in</strong> chronically ventilated preterm lambs. Am J Respir Crit Care Med.2005;172(7):899-906.25. Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restoreslung structure <strong>in</strong> eNOS-deficient mice recover<strong>in</strong>g from neonatal hypoxia. Am J Physiol LungCell Mol Physiol. 2006;291(1):L119-127.26. McCurn<strong>in</strong> DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, et al.Inhaled NO improves early pulmonary function and modifies lung growth and elast<strong>in</strong>deposition <strong>in</strong> a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell MolPhysiol. 2005;288(3):L450-459.27. Mart<strong>in</strong> RJ, Mhanna MJ, Haxhiu MA. The role of endogenous and exogenous nitric oxide onairway function. Sem<strong>in</strong> Per<strong>in</strong>atol. 2002;26(6):432-438.28. Cotten RB, Sundell HW, Zeld<strong>in</strong> DC, Morrow JD, Roberts LJ, Haz<strong>in</strong>ski TA, et al. Inhaled nitircoxide attenuates hyperoxic lung <strong>in</strong>jury <strong>in</strong> lambs. Pediatr Res. 2006;59:142-146.29. Roberts J, F<strong>in</strong>eman J, Mor<strong>in</strong> F, Shaul P, Rimar S, Schreiber M, et al. Inhaled nitric oxide andpersistent pulmonary hypertension of the newborn. N Engl J Med. 1997;336:605-610.30. Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dosenitric oxide therapy for persistent pulmonary hypertension of the newborn. Cl<strong>in</strong>ical InhaledNitric Oxide Research Group. N Engl J Med. 2000;342(7):469-474.1/18/2011 33
31. Group TNINOS. Inhaled nitric oxide <strong>in</strong> full-term and nearly full-term <strong>in</strong>fants with hypoxicrespiratory failure. New Engl J Med. 1997;336:597-604.32. K<strong>in</strong>sella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S, et al. Inhaled nitricoxide <strong>in</strong> premature neonates with severe hypoxaemic respiratory failure: a randomisedcontrolled trial. Lancet. 1999;354(9184):1061-1065.33. Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitricoxide for preterm <strong>in</strong>fants with severe respiratory failure. New Engl J Med. 2005;353:13-22.34. Field D, Elbourne D, Truesdale A, Grieve R, Hardy P, Fenton AC, et al. Neonatal VentilationWith Inhaled Nitric Oxide Versus Ventilatory Support Without Inhaled Nitric Oxide for PretermInfants With Severe Respiratory Failure: the INNOVO multicentre randomised controlled trial(ISRCTN 17821339). Pediatrics. 2005;115(4):926-936.35. Banks BA, Seri I, Ischiropoulos H, Merrill J, Rychik J, Ballard RA. Changes <strong>in</strong> oxygenation with<strong>in</strong>haled nitric oxide <strong>in</strong> severe bronchopulmonary dysplasia. Pediatrics. 1999;103(3):610-618.36. Clark PL, Ekekezie II, Kaftan HA, Castor CA, Truog WE. Safety and efficacy of nitric oxide <strong>in</strong>chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2002;86(1):F41-45.37. Schreiber MD, G<strong>in</strong>-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide <strong>in</strong>premature <strong>in</strong>fants with the respiratory distress syndrome. N Engl J Med. 2003;349(22):2099-2107.38. K<strong>in</strong>sella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early <strong>in</strong>haled nitricoxide therapy <strong>in</strong> premature newborns with respiratory failure. N Engl J Med. 2006;355(4):354-364.39. Ballard PL, Gonzales LW, God<strong>in</strong>ez RI, God<strong>in</strong>ez MH, Savani RC, McCurn<strong>in</strong> DC, Gibson LL,Yoder BA, Kerecman JD, Grubb PH, Shaul PW. Surfactant composition and function <strong>in</strong> aprimate model of <strong>in</strong>fant chronic lung disease: effects of <strong>in</strong>haled nitric oxide. Pediatr Res.2006;59(1):157-162.40. Hibbs AM, Black D, Palermo L, Cnaan A, Luan X, Walsh M, Ballard R. Account<strong>in</strong>g forMultiples Births <strong>in</strong> Neonatal and Per<strong>in</strong>atal Trials – Systematic Review and Case Study. JPediatrics. 2010 Feb; 156(2):202-8.41. Zupancic JAF, Hibbs AM, Palermo L, Truog WE, Cnaan A, Black D, Ballard PL, Merrill JD,Wadl<strong>in</strong>ger SR, Ballard RA. Economic Evaluation of Inhaled Nitric Oxide <strong>in</strong> Preterm InfantsUndergo<strong>in</strong>g Mechanical Ventilation. Pediatrics. 2009 Nov; 124(5):e1001-6.42. Hibbs AM, Walsh MC, Mart<strong>in</strong> RJ, Truog WE, Lorch SA, Alessandr<strong>in</strong>i E, Cnaan A, Palermo L,Wadl<strong>in</strong>ger SR, Coburn CE, Ballard PL, Ballard RA. One-year respiratory outcomes of preterm<strong>in</strong>fants enrolled <strong>in</strong> the Nitric Oxide (to prevent) Chronic Lung Disease trial. J Pediatr.2008;153(4):525-529.43. Walsh MC, HIbbs AM, Mart<strong>in</strong> C, Cnaan A, Keller RL, Vitt<strong>in</strong>ghoff E, Mart<strong>in</strong> R, Truog W, BallardP, Zadell A, Wadl<strong>in</strong>ger S, Coburn C, Ballard R. Two-Year Neurodevelopmental Outcomes ofVentilated Preterm Infants Treated with Inhaled Nitric Oxide(The NO CLD Trial). J Pediatr.2010 Apr: 156(4) 556-561.44. Keller RL, Walsh M, Vitt<strong>in</strong>ghoff E, Palermo L, Ballard PL, Ballard RA. Response to <strong>in</strong>halednitric oxide (iNO) and neurodevelopmental impairment (NDI) <strong>in</strong> NO CLD. E-PAS. 2009;2155.7.45. Zhu Y, Guo C, Cao L, Gong X, Wang C, Sun B. Different effects of surfactant and <strong>in</strong>halednitric oxide <strong>in</strong> modulation of <strong>in</strong>flammatory <strong>in</strong>jury <strong>in</strong> ventilated piglet lungs. Pulm PharmacolTher. 2005;18(4):303-313.46. Matalon S, DeMarco V, Haddad IY, Myles C, Skimm<strong>in</strong>g JW, Schurch S, et al. Inhaled nitricoxide <strong>in</strong>jures the pulmonary surfactant system of lambs <strong>in</strong> vivo. Am J Physiol. 1996;270(2 Pt1):L273-280.47. Haddad IY, Zhu S, Crow J, Barefield E, Gadilhe T, Matalon S. Inhibition of alveolar type II cellATP and surfactant synthesis by nitric oxide. Am J Physiol. 1996;270(6 Pt 1):L898-906.48. Ayad O, Wong HR. Nitric oxide decreases surfactant prote<strong>in</strong> A gene expression <strong>in</strong> H441 cells.Crit Care Med. 1998;26(7):1277-1282.49. Bhandari V, Johnson L, Smith-Kirw<strong>in</strong> S, Vigliotta G, Funanage V, Chander A. Hyperoxia andnitric oxide reduce surfactant components (DSPC and surfactant prote<strong>in</strong>s) and <strong>in</strong>creaseapoptosis <strong>in</strong> adult and fetal rat type II pneumocytes. Lung. 2002;180(6):301-317.1/18/2011 34
50. Sal<strong>in</strong>as D, Sparkman L, Berhane K, Boggaram V. Nitric oxide <strong>in</strong>hibits surfactant prote<strong>in</strong> Bgene expression <strong>in</strong> lung epithelial cells. Am J Physiol Lung Cell Mol Physiol.2003;285(5):L1153-1165.51. Sparkman L, Boggaram V. Nitric oxide <strong>in</strong>creases IL-8 gene transcription and mRNA stability toenhance IL-8 gene expression <strong>in</strong> lung epithelial cells. Am J Physiol Lung Cell Mol Physiol.2004;287(4):L764-773.52. Lee JW, Gonzalez RF, Chap<strong>in</strong> CJ, Busch J, F<strong>in</strong>eman JR, Gutierrez JA. Nitric oxide decreasessurfactant prote<strong>in</strong> gene expression <strong>in</strong> primary cultures of type II pneumocytes. Am J PhysiolLung Cell Mol Physiol. 2005;288(5):L950-957.53. Ballard PL, Merrill JD, Truog WE, God<strong>in</strong>ez RI, God<strong>in</strong>ez MH, McDevitt TM, N<strong>in</strong>g Y, Golombek,SG, Parton LA, Luan X, Cnaan A, Ballard RA. Surfactant function and composition <strong>in</strong>premature <strong>in</strong>fants treated with <strong>in</strong>haled nitric oxide. Pediatrics. 2007;120(2):346-353.54. Ballard RA, Ballard PL, Cnaan A, P<strong>in</strong>to-Mart<strong>in</strong> J, Davis DJ, Padbury JF,Phibbs RH, Parer JT,Hart MC, Mann<strong>in</strong>o FL, Sawai SK. Antenatal thyrotrop<strong>in</strong>-releas<strong>in</strong>g hormone to prevent lungdisease <strong>in</strong> preterm <strong>in</strong>fants. New Engl J Med. 1998;338:493-498.55. Keller RL, Vitt<strong>in</strong>ghoff E, Palermo L, Ballard RA, Ballard PL, Truog WE. Response to <strong>in</strong>halednitric oxide (INO): further analysis of the NO CLD trial. E-PAS. 2009;4515.4.56. Fisher L, Dixon DO, Herson J, Frankowski RK, Hearron MS, Peace KE. Intention to treat <strong>in</strong>cl<strong>in</strong>ical trials. In statistical issues <strong>in</strong> drug research and development. New York: MarcelDekker; 1990.57. Liang KY, Zeger SL. Longitud<strong>in</strong>al data analysis us<strong>in</strong>g generalized l<strong>in</strong>ear models. Biometrika.1986;73:13-22.58. Rub<strong>in</strong> DB. Multiple Imputation for Nonresponse <strong>in</strong> Surveys. New York: John Wiley & Sons;1987.59. Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics <strong>in</strong> medic<strong>in</strong>e--report<strong>in</strong>g ofsubgroup analyses <strong>in</strong> cl<strong>in</strong>ical trials. N Engl J Med. 2007;357(21):2189-2194.60. F<strong>in</strong>e JP, Gray RJ. A proportional hazards model for the subdistribution of a compet<strong>in</strong>g risk.Journal of the American Statistical Association 1999;94(446):496-509W.61. R Development Core Team. R: A language and environment for statistical comput<strong>in</strong>g. RFoundation for Statistical Comput<strong>in</strong>g. In. Vienna, Austria: ISBN 3-900051-07-0, URLhttp://www.r-project.org.; 2008.1/18/2011 35