You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
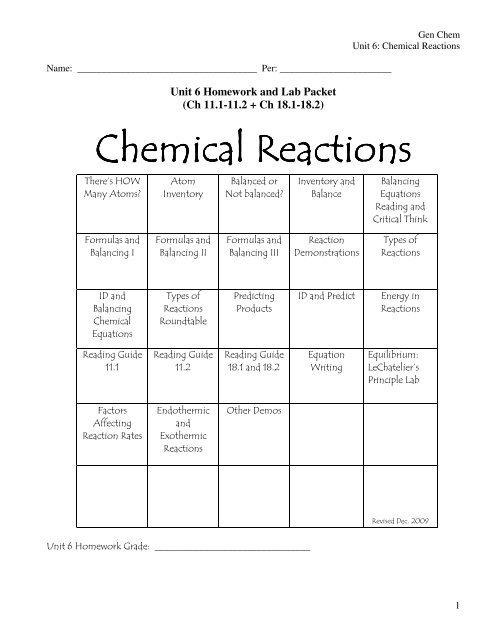
Name: _____________________________________ Per: _______________________<strong>Unit</strong> 6 <strong>Homework</strong> and Lab <strong>Packet</strong>(Ch 11.1-11.2 + Ch 18.1-18.2)Gen Chem<strong>Unit</strong> 6: Chemical Reactions !" #$" $" $" %& '%!"& '"(("%()"*+*)"*+,)"*-+**-+,!"!""./(& /$' 0%%+,1123 4 5#).666666666666666666666666666666661
Gen Chem<strong>Unit</strong> 6: Chemical Reactions Understand and apply knowledge of chemical reactions. ! "#$ ! "#% & "#' ( ! " # ) " #* "*#+ , !"* #-../ . 01&• Conservation of matter• Common reactions• Types of reactions• Common reactions in living systems2 • 3 45 4• 3 4• 343 42
Gen Chem<strong>Unit</strong> 6: Chemical Reactions . 5 6 .."5# $.". $ # ."6 # . $ 7 %5 7 .". 5 $ # .". % # $.". $ # 1 8 6 5)1 '1 % 7 $1 7 8 "1 % # $ 8 $65 % 6 • .7 " #• 1 9"9 #3
Gen Chem<strong>Unit</strong> 6: Chemical Reactions. :; →: < 4 =6> ?: $6 ; 5 →(65 $ < 4 =6$> 6 5 ?$8;%5 →8 $ % ;%5 < 4 =6> ?8 5 % . ;%6 ? . 6 < 4
' "1 % # $ ;."5# →"5# $ ;.1 % < 4=6> 1 % . 5 ?( "1 % # $ ;$."5# → "5# $ ;$.1 % < 4 =6> 1 % . 5 ?) %1 ; 58→18 % ;5 < 4 =6> 1 5 8 ?Gen Chem<strong>Unit</strong> 6: Chemical Reactions* "6 $ # ;@ . % →. % ; @6 $ < 4 =6> 6 $ @ . % ?5
Gen Chem<strong>Unit</strong> 6: Chemical Reactions 3>. A4BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB."5# ; 5... ; 5 < 4BBBBBBBBBBBBBBBBBBBB "1 % # $ ;$
- 2 @. $ @.;
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsInformation: Subscripts and CoefficientsA subscript is a small number that tells you how many atoms are in a compound. For example,in CaCl 2 the two is the subscript and it tells us that there are two chloride ions bonded to onecalcium.A coefficient tells also tells us how many atoms or compounds there are, but in a different way.For example in the expression “3 H 2 O” the three is the coefficient. The three tells us that thereare three molecules of water present. In the expression “3 H 2 O” there are a total of 6 hydrogenatoms and 3 oxygen atoms.Critical Thinking Questions1. Verify that in 4Ca 3 (PO 4 ) 2 there are 32 oxygen atoms present.2. How many oxygen atoms are in each of the following:_____ a) Al 2 O 3 _____ b) 3 Na 2 O _____ c) 4 Na 2 SO 4 _____ d) 5 Mg(NO 3 ) 28
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsInformation: How To Balance EquationsConsider the reaction of sodium and chlorine to produce sodium chloride from question one:Na + Cl 2 NaClRemember that when chlorine is by itself it is always written as Cl 2 . On the reactant side of thereaction (left side) there are a total of two atoms of chlorine, but on the product side there is onlyone atom of chlorine. Atoms cannot simply disappear. In order for the equation to make sense,we need to balance the equation. This can be done, first, by adding a “2” to the product side:Na + Cl 2 2 NaClNow the equation reads that one atom of Na reacts with one molecule of Cl 2 to produce twounits of NaCl. However, now the Na atoms are not balanced because there is one atom of thereactant side, but two atoms of Na on the product side. This can be fixed by adding anothertwo:Let us consider another example:2 Na + Cl 2 2 NaClCa 3 N 2 + NaCl CaCl 2 + Na 3 NNotice that none of the atoms are “balanced”. There are three calcium atoms on one side andonly one on the other. There are two nitrogen atoms on one side and one on the other. Howcan we fix this? Begin by “balancing” one atom at a time:1. First, let’s balance the calcium atoms by placing a three in front of CaCl 2 :Ca 3 N 2 + NaCl 3 CaCl 2 + Na 3 N2. Next, let’s balance the nitrogen atoms, by placing a 2 in front of Na 3 N:Ca 3 N 2 + NaCl 3 CaCl 2 + 2 Na 3 N3. Now we will balance the sodium atoms by placing a 6 in front of NaCl.Ca 3 N 2 + 6 NaCl 3 CaCl 2 + 2 Na 3 N4. Finally, examine the chlorine atoms and notice that they are already balanced.5. Double check each atom to make sure there are equal numbers of each onboth sides of the equation.When balancing equations you NEVER change subscripts. Only change the coefficients.9
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsCritical Thinking Questions3. Which of the following equations are properly and completely balanced? (Circle as many asapply.)A) 2 NaCl + Cu CuCl 2 + NaB) C 3 H 8 + 3 O 2 3 CO 2 + H 2 OC) MgCl 2 + F 2 MgF 2 + Cl 2D) FeCl 2 + O 2 2 FeO + Cl 24. Balance each of the following equations by inserting the correct coefficient in each blank.Remember to balance one atom at a time. If the number “one” belongs in the blank you mayeither leave it blank or insert the number one.A) _____ CaCl 2 + _____ Li 2 O _____ CaO + _____ LiClB) _____ Al 2 S 3 + _____ Cu _____ CuS + _____ AlC) _____ Na 3 P + _____ MgBr 2 _____ Mg 3 P 2 + _____ NaBr5. Here are the answers to question 4. Double check your answers to question 4:A) CaCl 2 + Li 2 O CaO + 2 LiClB) Al 2 S 3 + 3 Cu 3 CuS + 2 AlC) 2 Na 3 P + 3 MgBr 2 Mg 3 P 2 + 6 NaBr10
Information: Balancing Equations Containing Polyatomic IonsGen Chem<strong>Unit</strong> 6: Chemical ReactionsWhen an equation contains polyatomic ions, it may look a little more difficult to balance. Butbalancing an equation containing polyatomic ions is really not much different from the ones youjust did. In the equations above, you kept in mind that you must balance only one atom at atime. With polyatomic ions, keep in mind that you balance one polyatomic ion at a time. Forexample, consider the following unbalanced equation.Na 2 SO 4 + Ca 3 (PO 4 ) 2 Na 3 PO 4 + CaSO 4The first thing you should do is take note of which atoms/ions are not balanced. Keep thepolyatomic ions together. In other words, do not balance the phosphorus and oxygen atomsseparately; instead, balance the phosphate ions on each side of the reaction. Follow thesesteps:1. Balance the sodium so that there are 6 sodium atoms on each side of thereaction:3 Na 2 SO 4 + Ca 3 (PO 4 ) 2 2 Na 3 PO 4 + CaSO 42. Now there are three sulfate ions on the left and one on the right. Add a threeto the right.3 Na 2 SO 4 + Ca 3 (PO 4 ) 2 2 Na 3 PO 4 + 3 CaSO 43. Take inventory of all the atoms and ions to make sure they are balanced. Theequation is now balanced.11
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsCritical Thinking Questions6. Which of the following equations are properly balanced?A) Ba(NO 3 ) 2 + Fe 2 SO 4 2 FeNO 3 + BaSO 4B) 3 SrCl 2 + Al(NO 3 ) 3 3 Sr(NO 3 ) 2 + AlCl 3C) Ca 3 (PO 4 ) 2 + 3 Na 2 CO 3 2 Na 3 PO 4 + 3 CaCO 3D) Fe(NO 3 ) 2 + Mg Mg(NO 3 ) 2 + Fe7. Balance each of the following equations by inserting the correct coefficient in each blank.Remember to balance one atom/ion at a time. If the number “one” belongs in the blank youmay either leave it blank or insert the number one.A) _____ Ca(NO 3 ) 2 + _____ Na _____ NaNO 3 + _____ CaB) _____ Al 2 (CO 3 ) 3 + _____ MgCl 2 _____ AlCl 3 + _____ MgCO 3C) _____ Ba 3 (PO 4 ) 2 + _____ Na 2 CO 3 _____ Na 3 PO 4 + _____ BaCO 38. Make sure you got the following answers for question 6A) Ca(NO 3 ) 2 + 2 Na 2 NaNO 3 + CaB) Al 2 (CO 3 ) 3 + 3 MgCl 2 2 AlCl 3 + 3 MgCO 3C) Ba 3 (PO 4 ) 2 + 3 Na 2 CO 3 2 Na 3 PO 4 + 3 BaCO 3 12
! "3 ( ) $ * % + ' C < ! BBB % ; BBB BBB % C BBB:; BBB5. BBB:. ;BBB5 $ BBBA; BBB BBBA% ' BBB@. $ BBBB@.;BBB Gen Chem<strong>Unit</strong> 6: Chemical ReactionsBBBB81; BBBB BBB8 $ ;BBB1 3" 9#2 ! ( ;) ; * ;;+ ;; C ; 13
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsFormulas and Balancing IIDirections: Write the chemical formulas for the following compounds. After you have written theformulas and the equation, you must balance the equation.1. sodium chloride + silver nitrate sodium nitrate + silver chloride2. aluminum sulfate + calcium hydroxide aluminum hydroxide + calcium sulfate3. potassium chlorate potassium chloride + oxygen4. dicarbon hexahydride + oxygen carbon dioxide + water5. iron + oxygen iron (II) oxide6. lead (II) nitrate + potassium chromate lead (II) chromate + potassium nitrate7. sodium + water sodium hydroxide + hydrogen14
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsFormulas and Balancing IIIDirections: Write the chemical formulas for the following compounds. After you have written theformulas and the equation, you must balance the equation.1. iron + copper (II) sulfate iron (III) sulfate + copper2. silver nitrate + zinc zinc nitrate + silver3. aluminum + nitrogen aluminum nitrate4. iron (III) oxide + aluminum iron + aluminum oxide5. calcium carbonate calcium oxide + carbon dioxide15
Gen Chem<strong>Unit</strong> 6: Chemical Reactions #$%& ". 2 "#1"#D "$#1? "%#D? 6 2 ! E1"--#".1 % # (A5. A"--#".. # CA "."6 $ # # A"65#'".
Gen Chem<strong>Unit</strong> 6: Chemical Reactions#13 3/! < /! A8
Gen Chem<strong>Unit</strong> 6: Chemical Reactions. . 1 1 5< < G "(& ") ( 5. .,2-65 5. @ &- 7$ $ H %
Gen Chem<strong>Unit</strong> 6: Chemical Reactions D1? >& 6 $ ? D#1? 3 3/! < /! A8 19
D? 'CA65 '"--#.1 % $ ? /#D? 3 3/! < /! A8 8D? 'CA 6 . $ ' ."6 $ # $ ? 8#D? 3 ! < ! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBGen Chem<strong>Unit</strong> 6: Chemical Reactions20
Gen Chem<strong>Unit</strong> 6: Chemical Reactions *"3 45 4? < & 21
Gen Chem<strong>Unit</strong> 6: Chemical Reactions #%. E 2 ! & 2 + ++ "/! & &! I ! BBB;BBB BBB $ BBB5 BBB5;BBB $ BBB65;BBB5 1 % BBB6 1 % ;BBB5 % BBB8;BBB BBB8 $ 22
Gen Chem<strong>Unit</strong> 6: Chemical Reactions' BBB"6 $ # ;@ . % BBB. % ;BBB@6 $ ( BBB5 ;BBB6 BBB65 $ ) BBB. $ 5 ' "6 $ # $ BBB. ;BBB6 ;BBB5 ;BBB * BBB8;BBB.. BBB8. ;BBB. + BBB@. $ BBB@.;BBB C BBBA;BBB5. BBB5 ;BBBA. 23
Gen Chem<strong>Unit</strong> 6: Chemical Reactions#$,-"< ! 2 ! "1# "D# "1?# "D?# ".#BBBBB 5 5; BBBBB 6.; 6 $ 66 $ ; .BBBBB$ A; 5. A. ; 5 BBBBB% . ( 5 ( ; . ; 5 BBBBB' 65; 5 1 % 6 1 % ; 55BBBBB( "1 % # $ ; ."5# "5# $ ; .1 % BBBBB) 5 ; 5 BBBBB* . ) 5 ( ; 5 ; . BBBBB+ :; .1 % :1 % ; .BBBBBC @. $ @.; BBBBB 5 ; 8 8 $ ; 5 BBBBB ."5# ; 56 $ ."6 $ # ; 5524
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsBBBBB$ 6 ; . 6 . $BBBBB% 5 ; 6 65 $ BBBBB' .. ; . $ ; .BBBBB( .. ; 5 1 .1; 5.BBBBB) . 5 ( ; . ; 5 BBBBB* . ; 6< 6.;
Gen Chem<strong>Unit</strong> 6: Chemical Reactions #%% "D • - 62>@-6H=&• / 222 2 2 2
Gen Chem<strong>Unit</strong> 6: Chemical Reactions$ ; 2BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 3/! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB < /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB%; 2BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 3/! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB < /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB'; 2BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 3/! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB < /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB( 2BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 3! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB < /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB27
Gen Chem<strong>Unit</strong> 6: Chemical Reactions) ; 2BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 3/! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB < /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB*;"--# 2BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 3/! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB < /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB+E; 2BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 3/! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB < /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBC"--# 2BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 3/! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB < /! BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB28
Gen Chem<strong>Unit</strong> 6: Chemical Reactions''" #$% D 9J1 5 ; . BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB @; BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB$ 5 ; BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBD % 5. BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB' @< BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB( 8 $ BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB1? ) ; A BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB* >; .. BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB+ :; 5. BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBC @; $ BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBD? >5; 5 1 % BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB A8 ; . BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB$ 6.;
Gen Chem<strong>Unit</strong> 6: Chemical Reactions#'- BBBBB 6; . BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 5 BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB$ . $ 5 * ; BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB% 65; @8 BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB' >1 % ; . BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB) >; BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB* A; @ . $ BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB+ $ BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBC 6.; A BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB 5 ; < BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB .- BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB$ . % 5 * ; BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB% .1; BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB30
Gen Chem<strong>Unit</strong> 6: Chemical Reactions,%A&- CC@K /? //? "L5#/ /.///? //? "L5#/ /.//$CC@K(CC@K$CC@K%CC@K$CC@K31
Gen Chem<strong>Unit</strong> 6: Chemical Reactions$/? /CC@K/? "L5#7%CC@K/ (CC@K/.//%/? CC@K//? "L5#CC@K/ $CC@K/.//'/? /CC@K/? "L5#7 CC@K/ CC@K/.//32
Gen Chem<strong>Unit</strong> 6: Chemical Reactions(/? %CC@K/CC@K/? "L5#/ /.(CC@K//)/? CC@K/CC@K/? "L5#/ %CC@K/.//*/? /CC@K/? "L5#7)'@K/ 'CC@K/.//33
Gen Chem<strong>Unit</strong> 6: Chemical Reactions+/? ''C@K//? "L5#7%CC@K/ /.(CC@K//C/? (CC@K/CC@K/? "L5#/ $CC@K/.//34
Gen Chem<strong>Unit</strong> 6: Chemical Reactions%."//(/0//(123$///1)# )-"4D ? .& 2 ! $ +) 5! 4* 5& ! 4C 3& ! "--# 3 &" # 3 ! 1 1! ! 35
Gen Chem<strong>Unit</strong> 6: Chemical Reactions D ? .& 2 ! $$+ 3 4 BBBBBBBBBBBBBBBBBBBBBBBBBBBBBB BBBBBBBBBBBBBBBBBBBBBBBBBBBBBB BBBBBBBBBBBBBBBBBBBBBBBBBBBBBB BBBBBBBBBBBBBBBBBBBBBBBBBBBBBB BBBBBBBBBBBBBBBBBBBBBBBBBBBBBB$ 3& 4' 3 & 434 ! 2 ) 37 436
Gen Chem<strong>Unit</strong> 6: Chemical Reactions%."$/5(//5(1 > '% MN1 D M ND 7D , $ D % D 8*'' D8*'"O#+ 6+ 37
Gen Chem<strong>Unit</strong> 6: Chemical Reactions( D " #4/) 3 4* D =MN-P)/5(1" '%+7'''#+ >.9! 4C 1 '' 7''$5 !. 5 . $ 4 5 !4 3 !438
,-"3 ! 3 4"#QBBBBBBBBBBB"#QBBBBBBBBBBB"#QBBBBBBBBBB"!#QBBBBBBBBBB1 "--# . $1 %1 'A (3Gen Chem<strong>Unit</strong> 6: Chemical Reactions39
)1"--# *1E+ "--#C1 Gen Chem<strong>Unit</strong> 6: Chemical Reactions40
Gen Chem<strong>Unit</strong> 6: Chemical Reactions,-""7$'- . 6 < 7 8 ;$ ; 1.6 7 81.6 ; - "---# 2" #! ! 8 ;$ 1.6 7 81.6 ; >.9 ! - 8;$ 8 ;$ "# 12?/11 8 ;$ ; 1.6 7 81.6 ; - "#- ! # /!# !$# / >.9/! H ( CC& & CCA "--#.. R(5 . 5." A#
Gen Chem<strong>Unit</strong> 6: Chemical Reactions'"'0, # #"1 ."5 # ; ( "!#;%. 7 7"!#.. % "!#;(5 "## - "--#)C# $C 5.DJ$# &? %# &? '0$ , ## '6. # 5.DJ?'$0$ , ## C8"6$#$CC&6 # 'C2 CA@1.61?" #$# >% 7%'%5&& 2!&%# 2 2 7CCA8"6 $ # $ 2$7CCA@1.62%7%(A65"8"5# $ #.2 D 1 /! . . " #1$1%
.2 2$2% Gen Chem<strong>Unit</strong> 6: Chemical Reactions$ "'# 3 ! 4- 4# - ! 4'$# / >.9%# / 6.& '$'# 3 ! 8"6$#$4- 4/>.9(# 3 ! @1.64- 4/>.9)# / 65 - !443
!#%%- Gen Chem<strong>Unit</strong> 6: Chemical Reactions 2 1 & A & - 1 2 - / / S ? ! & 3 4 3 & 4$ 3 43 ! % 3 "A# 4' 5E 444
Gen Chem<strong>Unit</strong> 6: Chemical Reactions 7. , . A - A =CCA DCC7? 3 % "(A$AAC'A#$ ,C % 1 "C'A#7A < A ?&A' ? ?D2. A ?( ? 271E CC 'C& ,& &1E&2$ & &% ?& CCD & &272 >% 'C&
Gen Chem<strong>Unit</strong> 6: Chemical Reactions CCA &&,&? D $ A 2 7A % . 2& < JD2'0$ ACC BBBBBBBBBB BBBBBBBBBB. ? 2"# ? ?"A0#C'ACA$CA(CA') 0'89&1E7.
D Gen Chem<strong>Unit</strong> 6: Chemical Reactions - & =& 2 1 - & 2& 2& @ 1 & . H 5. H 2$ . BBBBBBBBBBBBBBBBBBBBBBBBBB BBBBBBBBBBBBBBBBBBBBBBBBBBB BBBBBBBBBBBBBBBBBBBBBBBBBBB% >& D 4/ ' >&23 4/( >&23 4/) , ? A%CA5." #BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB ? ACA5.' ." #BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB47
Gen Chem<strong>Unit</strong> 6: Chemical Reactions* D 24. "# + - 4/ 48
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsEndothermic and Exothermic ReactionsMany chemical reactions give off energy. Chemical reactions that release energy are called exothermicreactions. Some chemical reactions absorb energy and are called endothermic reactions. You will studyone exothermic and one endothermic reaction in this experiment.In Part I, you will study the reaction between citric acid solution and baking soda. An equation for thereaction is:H 3 C 6 H 5 O 7(aq) + 3 NaHCO 3(s) ⎯ → 3 CO 2(g) + 3 H 2 O (l) + Na 3 C 6 H 5 O 7(aq)In Part II, you will study the reaction between magnesium metal and hydrochloric acid. An equation forthis reaction is:Mg (s) + 2 HCl (aq) ⎯ → H 2(g) + MgCl 2(aq)Another objective of this experiment is for you to become familiar with using the LabPro data-collectionprogram on the computer. In this experiment, you will use the program to collect and display data as agraph or list, to examine your experimental data values on a graph, and to print graphs and data lists.MATERIALS Figure 1PROCEDURECBL SystemVernier Temperature ProbeVernier adapter cable10 mL pipet and pumpbalanceTwo test tubescitric acid, H 3 C 6 H 5 O 7 , solutionbaking soda, NaHCO 3hydrochloric acid, HCl, solutionmagnesium, Mg1. Obtain and wear goggles.Part I Citric Acid plus Baking Soda2. Plug the temperature probe should be connected to the computer and ready to use.3. Pipet out 10 mL of citric acid solution into your ignition tube and then place the ignition tube in a ringstand as shown in Figure 1. Place the temperature probe into the citric acid solution.4. Weigh out 3.00 g of solid baking soda on a piece of weighing paper.5. Observe your teacher on the steps to complete the following setup as the steps are different for acalculator and a computer. Set up the computer for one temperature probe and a temperaturecalibration.• Select SET UP PROBES from the MAIN MENU.49
Gen Chem<strong>Unit</strong> 6: Chemical Reactions• Enter “1” as the number of probes. Note: To enter a value, type the number on your calculator, thenpress ENTER .• Select TEMPERATURE from the SELECT PROBE menu.• Enter “1” as the channel number.• Select USE STORED from the CALIBRATION menu.6. Set up the computer for data collection.• Select COLLECT DATA from the MAIN MENU.• Select TIME GRAPH from the DATA COLLECTION menu.• Enter “6” as the time between samples, in seconds.• Enter “50” as the number of samples (the CBL will collect data for 5 minutes).• Press ENTER . Select USE TIME SETUP to continue. If you want to change the sample time or samplenumber, select MODIFY SETUP.• Enter “-10” as the minimum temperature (Ymin). To enter “-10”, use(—), not —.• Enter “40” as the maximum temperature (Ymax).• Enter “5” as the temperature increment (Yscl).7. When everything is ready, press ENTER on the calculator to begin data collection. After about 20seconds have elapsed, add the baking soda to the citric acid solution. Gently stir the solution with thetemperature probe to ensure good mixing. Don’t worry if the reaction foams over and out of the testtube, but keep the calculator and CBL device out of the way so there is no chance of an electricalshort. A real-time graph of temperature vs. time will be displayed on the computer screen during datacollection. Temperature readings (in °C) can also be monitored on the screen. When data collectionstops after 5 minutes, “SAMPLING” will change to “DONE” on the screen. A message will be displayedon the computer telling the data lists in which the time and temperature data are stored.8. Dispose of the reaction products as directed by your teacher.9. Press ENTER to display a graph of temperature vs. time on the computer screen. Examine the datapoints along the displayed curve using the mouseTI-83plus Calculators:Use the mouse to examine the data points along the curve. As you move the cursor right or left, thetime (X) and temperature (Y) values of each data point are displayed below the graph. Determine theinitial temperature, t 1 , and final (or minimum) temperature, t 2 . Record the temperature values inyour data table (round to the nearest 0.1°C).10. Make a sketch of the temperature vs. time graph in the observations section of the lab. Be sure to labelthe x and y axes with the appropriate units of time and temperature.50
Part II Hydrochloric Acid Plus MagnesiumGen Chem<strong>Unit</strong> 6: Chemical Reactions11. Press ENTER , then choose to repeat the data collection by selecting YES. Use the same Y-axis settingsas in Part I. Wait until Step 14 to begin collecting data.12. Measure out 10 mL of HCl solution into ignition tube. Place the temperature probe into the HClsolution. Note: The temperature probe must be in the HCl solution for at least 45 seconds before doingStep 15.13. Obtain a piece of magnesium metal from the teacher.14. Press ENTER to begin data collection. After about 20 seconds have elapsed, add the Mg to the HClsolution. Gently stir the solution with the temperature probe to ensure good mixing. Caution: Do notbreathe the vapors. “DONE” will appear on the CBL screen after data has been collected for a total of 5minutes.15. Dispose of the reaction products as directed by your teacher. Rinse the temperature probe.16. Press ENTER to display a graph of temperature vs. time on the screen. Examine the data points alongthe curve. Determine the initial temperature, t 1 , and the final (or maximum) temperature, t 2 . Recordthe temperature values in your data table (round to the nearest 0.1°C).17. Make a sketch of the temperature vs. time graph in the observations section of the lab. Besure to label the x and y axes with the appropriate units of time and temperature.OBSERVATIONSDescribe any chemical changes that you observe during the reaction of citric acid and baking soda:Describe any chemical changes that you observe during the reaction of hydrochloric acid andmagnesium metalDATA AND CALCULATIONSPart IPart IIFinal temperature, t 2 ________°C ________°CInitial temperature, t 1 ________°C ________°CTemperature change, ∆t∆t = t 2 - t 1________°C________°C51
Gen Chem<strong>Unit</strong> 6: Chemical ReactionsCaution: Do not do sketches until your calculator displays the final graph for each reaction!Analysis Questions1. Tell which reaction is exothermic. Explain.2. Which reaction had a negative ∆t value? Is the reaction endothermic or exothermic? Explain.3. For each reaction, describe three ways you could tell a chemical reaction was taking place.4. Which reaction took place at a greater rate? Explain your answer.Adapted from lab by:52
Gen Chem<strong>Unit</strong> 6: Chemical Reactions%! 8." %" 34 5 4347" # 345 434, $ , 34!9.3434343 453
Gen Chem<strong>Unit</strong> 6: Chemical Reactions."8# 34 34 3 4%24 34 3 & 4 34 3 454