Materials Measurement Phil Bartley Shelley Begley
Materials Measurement Phil Bartley Shelley Begley
Materials Measurement Phil Bartley Shelley Begley
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
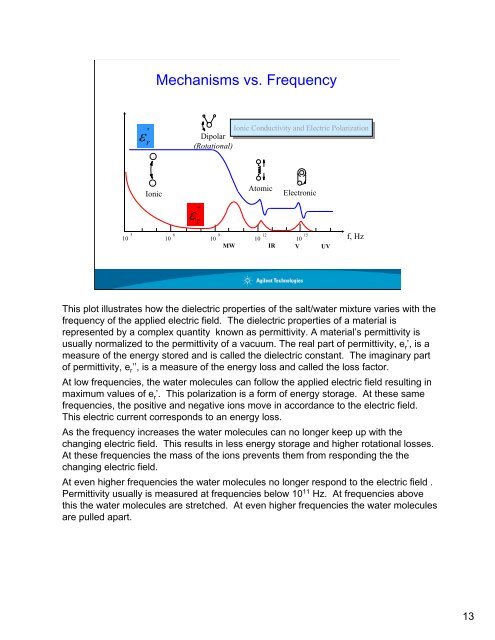
Mechanisms vs. Frequency'ε rIonic Ionic Conductivity and and Electric Electric PolarizationDipolar(Rotational)+++-Ionic''ε r-Atomic-Electronic10 3 10 6 10 9 10 12 10 15 f, HzMWIR V UVThis plot illustrates how the dielectric properties of the salt/water mixture varies with thefrequency of the applied electric field. The dielectric properties of a material isrepresented by a complex quantity known as permittivity. A material’s permittivity isusually normalized to the permittivity of a vacuum. The real part of permittivity, e r ’, is ameasure of the energy stored and is called the dielectric constant. The imaginary partof permittivity, e r ’’, is a measure of the energy loss and called the loss factor.At low frequencies, the water molecules can follow the applied electric field resulting inmaximum values of e r ’. This polarization is a form of energy storage. At these samefrequencies, the positive and negative ions move in accordance to the electric field.This electric current corresponds to an energy loss.As the frequency increases the water molecules can no longer keep up with thechanging electric field. This results in less energy storage and higher rotational losses.At these frequencies the mass of the ions prevents them from responding the thechanging electric field.At even higher frequencies the water molecules no longer respond to the electric field .Permittivity usually is measured at frequencies below 10 11 Hz. At frequencies abovethis the water molecules are stretched. At even higher frequencies the water moleculesare pulled apart.13