A LANTHANIDE LANTHOLOGY (.pdf) - Davidson Physics
A LANTHANIDE LANTHOLOGY (.pdf) - Davidson Physics A LANTHANIDE LANTHOLOGY (.pdf) - Davidson Physics
OXALATESAddition of oxalic acid, or an alkali oxalate or ammonium oxalate, to a neutral or slightlyacidic solution of a trivalent lanthanide will precipitate a hydrated oxalate. The deca-hydrate isobtained with La - Ho (and Y), an isomorphous series, whereas another series, the hexa-hydrate,tends to form for Er - Lu, the heavier lanthanides. In strongly acidic solutions the other anionpresent may be included in the solid brought down, chloro-oxalate from HCl for example, or aninsoluble acid salt be produced.The high insolubility of the hydrated oxalate in near-neutral solutions is used in thecommercial-scale preparation of high-purity derivatives of the lanthanides and yttrium.Calcination of the precipitate produces a pure oxide. This process is also used for the gravimetricanalysis of lanthanides. The oxalate is preferred to the carbonate because transition metals ionstend to be rejected and remain in solution. Physical occlusion of contaminant ions though can be aproblem.Thermal decomposition[1] of the light-Ln oxalates involves initially the steady loss of waterof hydration, from ambient up to≈ 250°C, but intermediate hydrates are not seen. With the heavylanthanides an intermediate dihydrate can be formed. The anhydrous oxalates are not stable and thefirst clearly identifiable species is the dioxomonocarbonate, Ln 2 O 2 CO 3 , containing the stable(LnO) n grouping seen in many Ln compounds.Ln 2 (C 2 O 4 ) 3 .nH 2 O => Ln 2 (C 2 O 4 ) 3 =>[? Ln 2 (CO 3 ) 3 => Ln 2 O(CO 3 ) 2 ?] =>=> Ln 2 O 2 CO 3 => Ln 2 O 3The temperatures corresponding to the various transitions depend on the surroundings, thepresence of CO 2 delays decomposition. In addition localized reducing conditions can cause spotformation of carbon. This can give a gray cast to the resulting oxide product if the C is not burnt offduring the final stages of the decomposition.Ln 2 (C 2 O 4 ) 3 => Ln 2 O 2 CO 3 + 2CO 2 + 3CO2CO => CO 2 + CWith cerium, oxidation of Ce(III) to Ce(IV) starts at low temperatures, ≈300 °C,because of the formation of the oxide, CeO 2 , no intermediate stages are seen.and,[1] Thermal Analysis of the Oxalate Hexahydrates and Decahydrates of Yttrium and the Lanthanide Elements,M.J. Fuller and J. Pinkstone, J. Less-Common Metals, 1980, 70, 12717
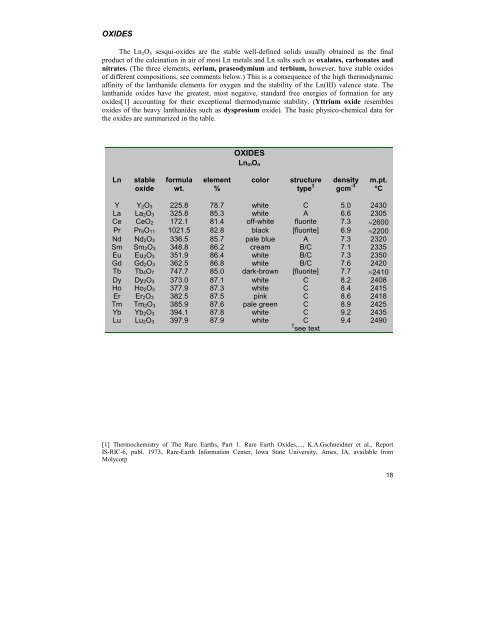
OXIDESThe Ln 2 O 3 sesqui-oxides are the stable well-defined solids usually obtained as the finalproduct of the calcination in air of most Ln metals and Ln salts such as oxalates, carbonates andnitrates. (The three elements, cerium, praseodymium and terbium, however, have stable oxidesof different compositions, see comments below.) This is a consequence of the high thermodynamicaffinity of the lanthanide elements for oxygen and the stability of the Ln(III) valence state. Thelanthanide oxides have the greatest, most negative, standard free energies of formation for anyoxides[1] accounting for their exceptional thermodynamic stability. (Yttrium oxide resemblesoxides of the heavy lanthanides such as dysprosium oxide). The basic physico-chemical data forthe oxides are summarized in the table.OXIDESLn mO nLnstableoxideformulawt.element%colorstructuretype †densitygcm -3m.pt.°CY Y 2O 3 225.8 78.7 white C 5.0 2430La La 2O 3 325.8 85.3 white A 6.6 2305Ce CeO 2 172.1 81.4 off-white fluorite 7.3 ≈2600Pr Pr 6O 11 1021.5 82.8 black [fluorite] 6.9 ≈2200Nd Nd 2O 3 336.5 85.7 pale blue A 7.3 2320Sm Sm 2O 3 348.8 86.2 cream B/C 7.1 2335Eu Eu 2O 3 351.9 86.4 white B/C 7.3 2350Gd Gd 2O 3 362.5 86.8 white B/C 7.6 2420Tb Tb 4O 7 747.7 85.0 dark-brown [fluorite] 7.7 ≈2410Dy Dy 2O 3 373.0 87.1 white C 8.2 2408Ho Ho 2O 3 377.9 87.3 white C 8.4 2415Er Er 2O 3 382.5 87.5 pink C 8.6 2418Tm Tm 2O 3 385.9 87.6 pale green C 8.9 2425Yb Yb 2O 3 394.1 87.8 white C 9.2 2435Lu Lu 2O 3 397.9 87.9 white C 9.4 2490† see text[1] Thermochemistry of The Rare Earths, Part 1. Rare Earth Oxides,..., K.A.Gschneidner et al., ReportIS-RIC-6, publ. 1973, Rare-Earth Information Center, Iowa State University, Ames, IA, available fromMolycorp18
- Page 2 and 3: ALANTHANIDELANTHOLOGYPart II, M - Z
- Page 6 and 7: Compounds of the perovskite, ABO 3
- Page 8 and 9: METALSThe lanthanides, when prepare
- Page 10: METALSMetallo-thermic oxide-reducti
- Page 13 and 14: MONAZITEMonazite, a light-lanthanid
- Page 15 and 16: NEODYMIUMNeodymium is the third mos
- Page 18 and 19: [2] Preparation, Phase Equilibria,
- Page 20 and 21: NOMENCLATURE58 - 71; the term is in
- Page 24 and 25: OXIDESCalcination in air for the th
- Page 26 and 27: OXIDESFurthermore oxides with Ln IV
- Page 28 and 29: OXYCHLORIDESThermal decomposition o
- Page 30 and 31: OXYSULFIDESAll the elements of the
- Page 32 and 33: PEROVSKITESA very wide range of mat
- Page 34 and 35: PHOSPHATESThe LnPO 4 compounds can
- Page 36 and 37: PRASEODYMIUMtransport of Pr happens
- Page 38 and 39: RESOURCESFor significant resources
- Page 40 and 41: RESOURCESSignificant new resources
- Page 42 and 43: SAMARIUMSamarium metal is made dire
- Page 44 and 45: SILICATESWithin the binary Ln 2 O 3
- Page 46 and 47: SOLVENT EXTRACTIONSome text books s
- Page 48 and 49: SULFATESLanthanide sulfates can be
- Page 50 and 51: SULFIDESThe thermochernistry of CeS
- Page 52 and 53: THULIUMThulium, the rarest of the "
- Page 54 and 55: TITANATES, TITANIUM DIOXIDELanthani
- Page 56 and 57: YTTERBIUMIn broad chemical behavior
- Page 58 and 59: YTTRIUMCompoundIdealFormulaFormula
- Page 60 and 61: YTTRIUM OXIDEThe very stable oxide,
- Page 62 and 63: YTTRIUM OXIDEThe widespread introdu
OXIDESThe Ln 2 O 3 sesqui-oxides are the stable well-defined solids usually obtained as the finalproduct of the calcination in air of most Ln metals and Ln salts such as oxalates, carbonates andnitrates. (The three elements, cerium, praseodymium and terbium, however, have stable oxidesof different compositions, see comments below.) This is a consequence of the high thermodynamicaffinity of the lanthanide elements for oxygen and the stability of the Ln(III) valence state. Thelanthanide oxides have the greatest, most negative, standard free energies of formation for anyoxides[1] accounting for their exceptional thermodynamic stability. (Yttrium oxide resemblesoxides of the heavy lanthanides such as dysprosium oxide). The basic physico-chemical data forthe oxides are summarized in the table.OXIDESLn mO nLnstableoxideformulawt.element%colorstructuretype †densitygcm -3m.pt.°CY Y 2O 3 225.8 78.7 white C 5.0 2430La La 2O 3 325.8 85.3 white A 6.6 2305Ce CeO 2 172.1 81.4 off-white fluorite 7.3 ≈2600Pr Pr 6O 11 1021.5 82.8 black [fluorite] 6.9 ≈2200Nd Nd 2O 3 336.5 85.7 pale blue A 7.3 2320Sm Sm 2O 3 348.8 86.2 cream B/C 7.1 2335Eu Eu 2O 3 351.9 86.4 white B/C 7.3 2350Gd Gd 2O 3 362.5 86.8 white B/C 7.6 2420Tb Tb 4O 7 747.7 85.0 dark-brown [fluorite] 7.7 ≈2410Dy Dy 2O 3 373.0 87.1 white C 8.2 2408Ho Ho 2O 3 377.9 87.3 white C 8.4 2415Er Er 2O 3 382.5 87.5 pink C 8.6 2418Tm Tm 2O 3 385.9 87.6 pale green C 8.9 2425Yb Yb 2O 3 394.1 87.8 white C 9.2 2435Lu Lu 2O 3 397.9 87.9 white C 9.4 2490† see text[1] Thermochemistry of The Rare Earths, Part 1. Rare Earth Oxides,..., K.A.Gschneidner et al., ReportIS-RIC-6, publ. 1973, Rare-Earth Information Center, Iowa State University, Ames, IA, available fromMolycorp18