Modelling reactive distillation
Modelling reactive distillation
Modelling reactive distillation
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
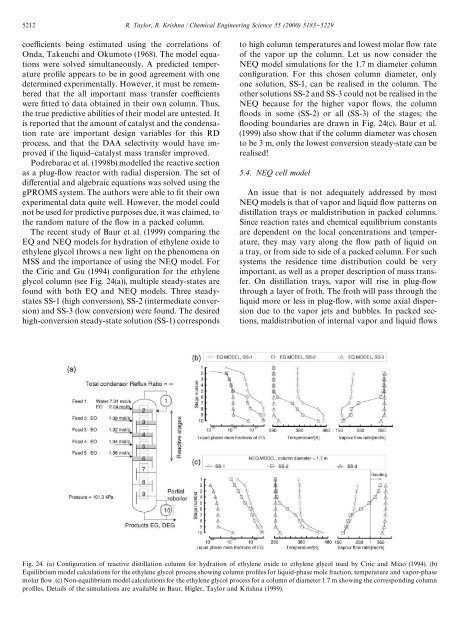
5212 R. Taylor, R. Krishna / Chemical Engineering Science 55 (2000) 5183}5229coe$cients being estimated using the correlations ofOnda, Takeuchi and Okumoto (1968). The model equationswere solved simultaneously. A predicted temperaturepro"le appears to be in good agreement with onedetermined experimentally. However, it must be rememberedthat the all important mass transfer coe$cientswere "tted to data obtained in their own column. Thus,the true predictive abilities of their model are untested. Itis reported that the amount of catalyst and the condensationrate are important design variables for this RDprocess, and that the DAA selectivity would have improvedif the liquid}catalyst mass transfer improved.Podrebarac et al. (1998b) modelled the <strong>reactive</strong> sectionas a plug-#ow reactor with radial dispersion. The set ofdi!erential and algebraic equations was solved using thegPROMS system. The authors were able to "t their ownexperimental data quite well. However, the model couldnot be used for predictive purposes due, it was claimed, tothe random nature of the #ow in a packed column.The recent study of Baur et al. (1999) comparing theEQ and NEQ models for hydration of ethylene oxide toethylene glycol throws a new light on the phenomena onMSS and the importance of using the NEQ model. Forthe Ciric and Gu (1994) con"guration for the ethyleneglycol column (see Fig. 24(a)), multiple steady-states arefound with both EQ and NEQ models. Three steadystatesSS-1 (high conversion), SS-2 (intermediate conversion)and SS-3 (low conversion) were found. The desiredhigh-conversion steady-state solution (SS-1) correspondsto high column temperatures and lowest molar #ow rateof the vapor up the column. Let us now consider theNEQ model simulations for the 1.7 m diameter columncon"guration. For this chosen column diameter, onlyone solution, SS-1, can be realised in the column. Theother solutions SS-2 and SS-3 could not be realised in theNEQ because for the higher vapor #ows, the column#oods in some (SS-2) or all (SS-3) of the stages; the#ooding boundaries are drawn in Fig. 24(c). Baur et al.(1999) also show that if the column diameter was chosento be 3 m, only the lowest conversion steady-state can berealised!5.4. NEQ cell modelAn issue that is not adequately addressed by mostNEQ models is that of vapor and liquid #ow patterns on<strong>distillation</strong> trays or maldistribution in packed columns.Since reaction rates and chemical equilibrium constantsare dependent on the local concentrations and temperature,they may vary along the #ow path of liquid ona tray, or from side to side of a packed column. For suchsystems the residence time distribution could be veryimportant, as well as a proper description of mass transfer.On <strong>distillation</strong> trays, vapor will rise in plug-#owthrough a layer of froth. The froth will pass through theliquid more or less in plug-#ow, with some axial dispersiondue to the vapor jets and bubbles. In packed sections,maldistribution of internal vapor and liquid #owsFig. 24. (a) Con"guration of <strong>reactive</strong> <strong>distillation</strong> column for hydration of ethylene oxide to ethylene glycol used by Ciric and Miao (1994). (b)Equilibrium model calculations for the ethylene glycol process showing column pro"les for liquid-phase mole fraction, temperature and vapor-phasemolar #ow. (c) Non-equilibrium model calculations for the ethylene glycol process for a column of diameter 1.7 m showing the corresponding columnpro"les. Details of the simulations are available in Baur, Higler, Taylor and Krishna (1999).