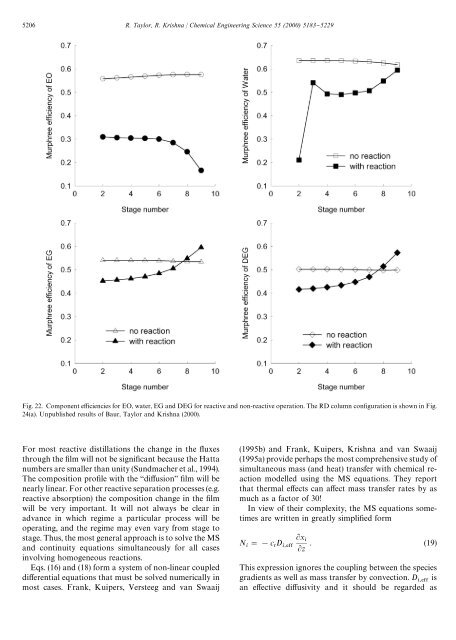
5206 R. Taylor, R. Krishna / Chemical Engineering Science 55 (2000) 5183}5229Fig. 22. Component e$ciencies for EO, water, EG and DEG for <strong>reactive</strong> and non-<strong>reactive</strong> operation. The RD column con"guration is shown in Fig.24(a). Unpublished results of Baur, Taylor and Krishna (2000).For most <strong>reactive</strong> <strong>distillation</strong>s the change in the #uxesthrough the "lm will not be signi"cant because the Hattanumbers are smaller than unity (Sundmacher et al., 1994).The composition pro"le with the `di!usiona "lm will benearly linear. For other <strong>reactive</strong> separation processes (e.g.<strong>reactive</strong> absorption) the composition change in the "lmwill be very important. It will not always be clear inadvance in which regime a particular process will beoperating, and the regime may even vary from stage tostage. Thus, the most general approach is to solve the MSand continuity equations simultaneously for all casesinvolving homogeneous reactions.Eqs. (16) and (18) form a system of non-linear coupleddi!erential equations that must be solved numerically inmost cases. Frank, Kuipers, Versteeg and van Swaaij(1995b) and Frank, Kuipers, Krishna and van Swaaij(1995a) provide perhaps the most comprehensive study ofsimultaneous mass (and heat) transfer with chemical reactionmodelled using the MS equations. They reportthat thermal e!ects can a!ect mass transfer rates by asmuch as a factor of 30!In view of their complexity, the MS equations sometimesare written in greatly simpli"ed formN "!c D x z . (19)This expression ignores the coupling between the speciesgradients as well as mass transfer by convection. D isan e!ective di!usivity and it should be regarded as
R. Taylor, R. Krishna / Chemical Engineering Science 55 (2000) 5183}5229 5207a function of the binary MS di!usion coe$cients,the mixture composition, and the mass transferrates themselves. However, if, as is usually done, weassume the e!ective di!usivity to be constant, then analyticalsolutions to Eqs. (18) and (19) may be derived.Indeed, the literature on mass transfer with chemicalreaction based on these two equations is vast and suchequations often are used. Results usually are expressed inthe formN "k E (x !x ), (20)where k is the mass transfer coe$cient and E is anenhancement factor that accounts for the in#uence ofchemical reaction on the molar #uxes. Among otherthings, enhancement factors depend quite strongly on thereaction kinetics; thus, there is no universal expressionfor E that can be used in all situations. Indeed, in manycases it is not even possible to obtain analytical expressionsfor the enhancement factor, even for the muchsimpli"edconstitutive relation given by Eq. (19).Danckwerts (1970) and Van Swaaij and Versteeg (1992)review the "eld of mass transfer with chemical reaction.Frank, Kuipers, Krishna and van Swaaij (1995a) citeother studies of non-isothermal mass transfer with simultaneouschemical reaction modelled using the simplerconstitutive relation given by Eq. (19). E!ective di!usivitymodels of this sort have been used with some successin the modelling of amine-based gas treating processes(see, for example, Cornelisse, Beenackers, van Beckum& van Swaaij, 1980; Carey, Hermes & Rochelle, 1991;Altiqi, Sabri, Bouhamra & Alper, 1994 and the extensivelists of references in these papers).A more rigorous approach to modelling mass transferin multicomponent systems is a!orded by the generalizedFick's law: xJ "!c D z , (21)where J is the molar di!usion #ux of speciesi (N "J #x N ) and the D are the Fick di!usion coe$cients for the multicomponent mixture. The Fickdi!usion coe$cients can be related to the binary MSdi!usion coe$cients; see Taylor and Krishna (1993) fordetails of this. Solutions of Eq. (21) and the continuityequation (18) are known for a few cases involving simultaneousdi!usion and reaction (see, for example, Toor,1965; Kenig & Gorak, 1995, 1997). In most cases ofpractical signi"cance Eq. (21) is just as di$cult to solve asthe full MS equations and it is necessary to employnumerical methods here as well.A rigorous approach to modelling heterogeneous systems(Fig. 5(b)) would involve a complete description ofmass transport to the catalyst and di!usion and reactioninside the catalyst particles, if the catalyst is porous, orreaction at the surface if it is not. In either case it isunnecessary to allow for reaction in the vapor or liquid"lms as described above.For all cases involving a heterogeneous reaction wemay model transport through the vapor}liquid interfaceusing the MS equations given above (assuming no reactionin the liquid "lm). We also need to model di!usion ofreactants to and products away from the solid catalystsurface using a separate set of di!usion equations. Forthose cases where the reaction takes place at the catalystsurface the boundary conditions at the liquid}solid interfacewill be determined by the reaction kinetics. However,if the reaction takes place inside a porous catalyst thenwe need to model di!usion and reaction inside the catalystas well as transport from the bulk liquid to/from thecatalyst surface.<strong>Modelling</strong> multicomponent mass transfer in porousmedia is complicated when the mean free path length ofthe molecules is of the order of magnitude of the porediameter. The di$culties posed by this case may becircumvented by a method originally introduced byMaxwell (1866) and developed further by Mason andco-workers (see Mason & Malinauskas, 1983). Maxwellsuggested that the porous material itself be describedas a supplementary `dusta species, consisting ofvery large molecules that are kept motionless bysome unspeci"ed external force. The Chapman}Enskogkinetic theory is then applied to the new pseudo-gasmixture, in which the interaction between the dust andgas molecules simulates the interaction between the solidmatrix and the gas species. In addition, one is no longerfaced with the problem of #ux and composition variationsacross a pore and problems related to catalystgeometry.The dusty #uid model as developed by Krishna andWesselingh (1997) is a modi"cation of the dusty gasmodel so as to be able to model liquid-phase di!usion inporous media. For a non-ideal mixture we have:x μ ¹ z # x ¹ < p z #x ηB D " x N !x N ! N , (22)c n c D where D is the e!ective Knudsen di!usion coe$cient forspecies i in the porous catalyst.The mass transfer rates are obtained by multiplyingthe #uxes by the interfacial area of the catalyst particles.This is not as straightforward, as it looks, since, dependingon the geometry of the catalyst, the cross-sectionalarea can change along the di!usion path. It is necessaryto take catalyst geometry into account in the solution ofthese equations.The dusty gas model is often used as the basis for thecalculation of a catalyst e!ectiveness factor (Jackson,1977). The extension to non-ideal #uids noted above hasnot been used as often, partly because of the greater
- Page 7 and 8: R. Taylor, R. Krishna / Chemical En
- Page 9 and 10: R. Taylor, R. Krishna / Chemical En
- Page 12 and 13: 5194 R. Taylor, R. Krishna / Chemic
- Page 14 and 15: 5196 R. Taylor, R. Krishna / Chemic
- Page 16 and 17: 5198 R. Taylor, R. Krishna / Chemic
- Page 18 and 19: 5200 R. Taylor, R. Krishna / Chemic
- Page 20 and 21: 5202 R. Taylor, R. Krishna / Chemic
- Page 22 and 23: 5204 R. Taylor, R. Krishna / Chemic
- Page 26 and 27: 5208 R. Taylor, R. Krishna / Chemic
- Page 28 and 29: 5210 R. Taylor, R. Krishna / Chemic
- Page 30 and 31: 5212 R. Taylor, R. Krishna / Chemic
- Page 32 and 33: 5214 R. Taylor, R. Krishna / Chemic
- Page 34 and 35: 5216 R. Taylor, R. Krishna / Chemic
- Page 36 and 37: 5218 R. Taylor, R. Krishna / Chemic
- Page 38 and 39: 5220 R. Taylor, R. Krishna / Chemic
- Page 40 and 41: 5222 R. Taylor, R. Krishna / Chemic
- Page 42 and 43: 5224 R. Taylor, R. Krishna / Chemic
- Page 44 and 45: 5226 R. Taylor, R. Krishna / Chemic
- Page 47: R. Taylor, R. Krishna / Chemical En