Dynamical Systems in Neuroscience:
Dynamical Systems in Neuroscience: Dynamical Systems in Neuroscience:
256 Excitabilitymembrane potential, V (mV)200-20-40Na+ inactivation gate, h-600.5I=-20h (V)I=10 0.60I=-15-80 mV rest 20 mV0.70 50 100 150-60 -40 -20 0 20 40time (ms)membrane potential, V (mV)00.10.20.30.4I=-10I=-15I=5I=0I=-5I=10h-nullcline1m (V)V-nullclinesFigure 7.32: Inhibition-induced spiking in the I Na,t -model. Parameters are the sameas in Fig. 5.6b, except g leak = 1.5 and m ∞ (V ) has k = 27.hyperpolarizationdeinactivationof I Naexcessof I Nanegativeinjecteddc-currentrestingpotentialinactivationof I Naactivationof I NaspikeFigure 7.33: Mechanism of inhibitioninducedspiking in the I Na,t -model.vation and the Na + conductance, g Na mh, increases. This leads to an imbalance of theinward current and to the generation of the first spike. During the spike, the currentinactivates completely, and the leak and negative injected currents repolarize and thenhyperpolarize the membrane. During the hyperpolarization, clearly seen in the figure,Na + current deinactivates and is ready for the generation of the next spike.To understand the dynamic mechanism of such an inhibition-induced spiking, weneed to consider the geometry of the nullclines of the model, depicted in Fig. 7.32,bottom. Notice how the position of the V -nullcline depends on I. Negative I shifts thenullcline down and leftward so that the vertex of its left knee, marked by a dot, movesto the left. As a result, the equilibrium of the system, which is the intersection of theV - and h-nullclines, moves toward the middle branch of the cubic V -nullcline. WhenI = −2, the equilibrium loses stability via supercritical Andronov-Hopf bifurcation,and the model exhibits periodic activity.Instead of the I Na,t -model, we could have used the I Na + I K -model or any othermodel with a low-threshold resonant gating variable. The key point here is not the ionicbasis of the spike-generation mechanism, but its dynamic attribute — the Andronov-Hopf bifurcation. Even the FitzHugh-Nagumo model (4.11, 4.12) can exhibit thisphenomenon; see Ex. 1.
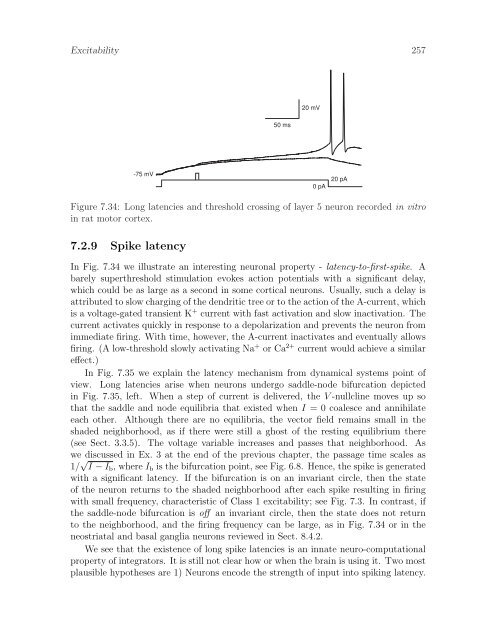
Excitability 25720 mV50 ms-75 mV0 pA20 pAFigure 7.34: Long latencies and threshold crossing of layer 5 neuron recorded in vitroin rat motor cortex.7.2.9 Spike latencyIn Fig. 7.34 we illustrate an interesting neuronal property - latency-to-first-spike. Abarely superthreshold stimulation evokes action potentials with a significant delay,which could be as large as a second in some cortical neurons. Usually, such a delay isattributed to slow charging of the dendritic tree or to the action of the A-current, whichis a voltage-gated transient K + current with fast activation and slow inactivation. Thecurrent activates quickly in response to a depolarization and prevents the neuron fromimmediate firing. With time, however, the A-current inactivates and eventually allowsfiring. (A low-threshold slowly activating Na + or Ca 2+ current would achieve a similareffect.)In Fig. 7.35 we explain the latency mechanism from dynamical systems point ofview. Long latencies arise when neurons undergo saddle-node bifurcation depictedin Fig. 7.35, left. When a step of current is delivered, the V -nullcline moves up sothat the saddle and node equilibria that existed when I = 0 coalesce and annihilateeach other. Although there are no equilibria, the vector field remains small in theshaded neighborhood, as if there were still a ghost of the resting equilibrium there(see Sect. 3.3.5). The voltage variable increases and passes that neighborhood. Aswe discussed in Ex. 3 at the end of the previous chapter, the passage time scales as1/ √ I − I b , where I b is the bifurcation point, see Fig. 6.8. Hence, the spike is generatedwith a significant latency. If the bifurcation is on an invariant circle, then the stateof the neuron returns to the shaded neighborhood after each spike resulting in firingwith small frequency, characteristic of Class 1 excitability; see Fig. 7.3. In contrast, ifthe saddle-node bifurcation is off an invariant circle, then the state does not returnto the neighborhood, and the firing frequency can be large, as in Fig. 7.34 or in theneostriatal and basal ganglia neurons reviewed in Sect. 8.4.2.We see that the existence of long spike latencies is an innate neuro-computationalproperty of integrators. It is still not clear how or when the brain is using it. Two mostplausible hypotheses are 1) Neurons encode the strength of input into spiking latency.
- Page 216 and 217: 206 Bifurcationsfast nullclineslow
- Page 218 and 219: 208 Bifurcationswith fast and slow
- Page 220 and 221: 210 BifurcationsSupercritical Andro
- Page 222 and 223: 212 BifurcationsK + conductance tim
- Page 224 and 225: 214 BifurcationsIn contrast, if the
- Page 226 and 227: 216 Bifurcationsinvariant circlesad
- Page 228 and 229: 218 Bifurcationsbifurcationssaddle-
- Page 230 and 231: 220 BifurcationsExercises1. (Transc
- Page 232 and 233: 222 Bifurcationsv 2v 11a-11-1Figure
- Page 234 and 235: 224 Bifurcations19. [M.S.] A leaky
- Page 236 and 237: 226 Excitabilityspike?spikerestrest
- Page 238 and 239: 228 ExcitabilityAlternatively, the
- Page 240 and 241: 230 Excitability50 ms 20 mV 1 ms100
- Page 242 and 243: 232 ExcitabilityClass 3 excitable n
- Page 244 and 245: 234 Excitability0.20.150.10.05I 1I
- Page 246 and 247: 236 Excitabilitysaddle-node bifurca
- Page 248 and 249: 238 Excitability(a) resting spiking
- Page 250 and 251: 240 Excitabilityproperties integrat
- Page 252 and 253: 242 ExcitabilityThe existence of fa
- Page 254 and 255: 244 Excitability1coincidencedetecti
- Page 256 and 257: 246 Excitabilityspikeexcitatory pul
- Page 258 and 259: 248 Excitability(g)9 ms(f)(e)(d)(c)
- Page 260 and 261: 250 Excitabilitysquid axonmodel0 mV
- Page 262 and 263: 252 Excitability(a) integrator(b) r
- Page 264 and 265: 254 Excitability(a) integrator(b) r
- Page 268 and 269: 258 Excitability0.1saddle-node bifu
- Page 270 and 271: 260 Excitability100 ms10 mVoscillat
- Page 272 and 273: 262 ExcitabilityBogdanov-Takens bif
- Page 274 and 275: 264 Excitabilitya pulse of current.
- Page 276 and 277: 266 Excitabilitymembrane potential
- Page 278 and 279: 268 Excitability(a)150100I K(M)(b)3
- Page 280 and 281: 270 Excitability50 mV300 msFigure 7
- Page 282 and 283: 272 Excitability20 mVADP100 msAHP-6
- Page 284 and 285: 274 ExcitabilityK + activation gate
- Page 286 and 287: 276 ExcitabilityBibliographical Not
- Page 288 and 289: 278 Excitability7. Show that the re
- Page 290 and 291: 280 Simple Modelsspikemembrane pote
- Page 292 and 293: 282 Simple Models1thresholdyz reset
- Page 294 and 295: 284 Simple Models1v reset =|b| 1/2b
- Page 296 and 297: 286 Simple Modelsbe an integrator o
- Page 298 and 299: 288 Simple Modelsintegrate-and-fire
- Page 300 and 301: 290 Simple Models(A) tonic spiking(
- Page 302 and 303: 292 Simple Modelsgeneral algorithm
- Page 304 and 305: 294 Simple Modelscha, cha — real
- Page 306 and 307: 296 Simple Modelslayer 5 neuronsimp
- Page 308 and 309: 298 Simple Modelsb=-240200saddle-no
- Page 310 and 311: 300 Simple Models(a)simple modellay
- Page 312 and 313: 302 Simple Models(a)burstingspiking
- Page 314 and 315: 304 Simple Models(a)dendriticspike2
Excitability 25720 mV50 ms-75 mV0 pA20 pAFigure 7.34: Long latencies and threshold cross<strong>in</strong>g of layer 5 neuron recorded <strong>in</strong> vitro<strong>in</strong> rat motor cortex.7.2.9 Spike latencyIn Fig. 7.34 we illustrate an <strong>in</strong>terest<strong>in</strong>g neuronal property - latency-to-first-spike. Abarely superthreshold stimulation evokes action potentials with a significant delay,which could be as large as a second <strong>in</strong> some cortical neurons. Usually, such a delay isattributed to slow charg<strong>in</strong>g of the dendritic tree or to the action of the A-current, whichis a voltage-gated transient K + current with fast activation and slow <strong>in</strong>activation. Thecurrent activates quickly <strong>in</strong> response to a depolarization and prevents the neuron fromimmediate fir<strong>in</strong>g. With time, however, the A-current <strong>in</strong>activates and eventually allowsfir<strong>in</strong>g. (A low-threshold slowly activat<strong>in</strong>g Na + or Ca 2+ current would achieve a similareffect.)In Fig. 7.35 we expla<strong>in</strong> the latency mechanism from dynamical systems po<strong>in</strong>t ofview. Long latencies arise when neurons undergo saddle-node bifurcation depicted<strong>in</strong> Fig. 7.35, left. When a step of current is delivered, the V -nullcl<strong>in</strong>e moves up sothat the saddle and node equilibria that existed when I = 0 coalesce and annihilateeach other. Although there are no equilibria, the vector field rema<strong>in</strong>s small <strong>in</strong> theshaded neighborhood, as if there were still a ghost of the rest<strong>in</strong>g equilibrium there(see Sect. 3.3.5). The voltage variable <strong>in</strong>creases and passes that neighborhood. Aswe discussed <strong>in</strong> Ex. 3 at the end of the previous chapter, the passage time scales as1/ √ I − I b , where I b is the bifurcation po<strong>in</strong>t, see Fig. 6.8. Hence, the spike is generatedwith a significant latency. If the bifurcation is on an <strong>in</strong>variant circle, then the stateof the neuron returns to the shaded neighborhood after each spike result<strong>in</strong>g <strong>in</strong> fir<strong>in</strong>gwith small frequency, characteristic of Class 1 excitability; see Fig. 7.3. In contrast, ifthe saddle-node bifurcation is off an <strong>in</strong>variant circle, then the state does not returnto the neighborhood, and the fir<strong>in</strong>g frequency can be large, as <strong>in</strong> Fig. 7.34 or <strong>in</strong> theneostriatal and basal ganglia neurons reviewed <strong>in</strong> Sect. 8.4.2.We see that the existence of long spike latencies is an <strong>in</strong>nate neuro-computationalproperty of <strong>in</strong>tegrators. It is still not clear how or when the bra<strong>in</strong> is us<strong>in</strong>g it. Two mostplausible hypotheses are 1) Neurons encode the strength of <strong>in</strong>put <strong>in</strong>to spik<strong>in</strong>g latency.