February 1, 2010, Home Health & Hospice Medicare A ... - CGS
February 1, 2010, Home Health & Hospice Medicare A ... - CGS February 1, 2010, Home Health & Hospice Medicare A ... - CGS
k. Payment Offset for Pass-Through Diagnostic RadiopharmaceuticalsEffective for nuclear medicine services furnished on and after April 1, 2009, CMS implemented a paymentoffset for pass-through diagnostic radiopharmaceuticals under the OPPS. As discussed in Transmittal 1702,CR 6416, issued March 13, 2009, pass-through payment for a diagnostic radiopharmaceutical is thedifference between the payment for the pass-through product and the payment for the predecessor productthat, in the case of diagnostic radiopharmaceuticals, is packaged into the payment for the nuclear medicineprocedure in which the diagnostic radiopharmaceutical is used. The MLN Matters ® article related to CR6416 is available at http://www.cms.hhs.gov/MLNMattersArticles/downloads/MM6416.pdf on the CMSWeb site.Effective April 1, 2009, the diagnostic radiopharmaceutical reported with HCPCS code C9247 (Iobenguane,I-123, diagnostic, per study dose, up to 10 millicuries) was granted pass-through status under the OPPS andassigned status indicator “G.” Therefore, in CY 2009, when HCPCS code C9247 is billed on the sameclaim with a nuclear medicine procedure, CMS reduces the amount of payment for the pass-throughdiagnostic radiopharmaceutical reported with HCPCS code C9247 by the corresponding nuclear medicineprocedure’s portion of its APC payment associated with “policy-packaged” drugs (offset amount) so noduplicate radiopharmaceutical payment is made.For CY 2010, HCPCS code C9247 is being replaced with HCPCS code A9582 (Iodine I-123 iobenguane,diagnostic, per study dose, up to 15 millicuries) and HCPCS code A9582 will continue on pass-throughstatus for CY 2010. Therefore, for CY 2010, HCPCS code A9582 will be assigned status indicator “G” andwill be subject to the pass-through payment offset for pass-through diagnostic radiopharmaceuticals. Theoffset will cease to apply when this diagnostic radiopharmaceutical expires from pass-through status.The “policy-packaged” portions of the CY 2010 APC payments for nuclear medicine procedures may befound on the CMS Web site at http://www.cms.hhs.gov/HospitalOutpatientPPS/APF/list.asp#TopOfPage inthe download file labeled 2010 OPPS Offset Amounts by APC.CY 2010 APCs to which nuclear medicine procedures are assigned and for which we expect a diagnosticradiopharmaceutical payment offset could be applicable in the case of a pass-through diagnosticradiopharmaceutical are displayed in the following table.Table 10-APCs to Which Nuclear Medicine Procedures are Assigned for CY 2010CY 2010 APC CY 2010 APC Title0307 Myocardial Positron Emission Tomography (PET) imaging0308 Non-Myocardial Positron Emission Tomography (PET) imaging0377 Level II Cardiac Imaging0378 Level II Pulmonary Imaging0389 Level I Non-imaging Nuclear Medicine0390 Level I Endocrine Imaging0391 Level II Endocrine Imaging0392 Level II Non-imaging Nuclear Medicine0393 Hematologic Processing & Studies0394 Hepatobiliary ImagingHome Health & Hospice February 1, 2010 31Medicare A Newsline Vol. 17, No. 5
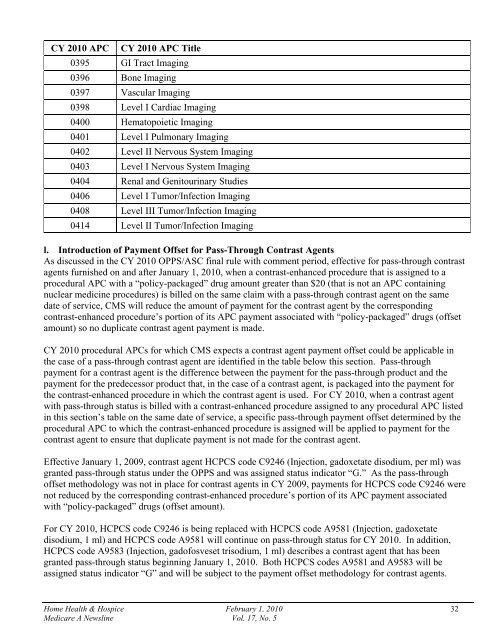
CY 2010 APCCY 2010 APC Title0395 GI Tract Imaging0396 Bone Imaging0397 Vascular Imaging0398 Level I Cardiac Imaging0400 Hematopoietic Imaging0401 Level I Pulmonary Imaging0402 Level II Nervous System Imaging0403 Level I Nervous System Imaging0404 Renal and Genitourinary Studies0406 Level I Tumor/Infection Imaging0408 Level III Tumor/Infection Imaging0414 Level II Tumor/Infection Imagingl. Introduction of Payment Offset for Pass-Through Contrast AgentsAs discussed in the CY 2010 OPPS/ASC final rule with comment period, effective for pass-through contrastagents furnished on and after January 1, 2010, when a contrast-enhanced procedure that is assigned to aprocedural APC with a “policy-packaged” drug amount greater than $20 (that is not an APC containingnuclear medicine procedures) is billed on the same claim with a pass-through contrast agent on the samedate of service, CMS will reduce the amount of payment for the contrast agent by the correspondingcontrast-enhanced procedure’s portion of its APC payment associated with “policy-packaged” drugs (offsetamount) so no duplicate contrast agent payment is made.CY 2010 procedural APCs for which CMS expects a contrast agent payment offset could be applicable inthe case of a pass-through contrast agent are identified in the table below this section. Pass-throughpayment for a contrast agent is the difference between the payment for the pass-through product and thepayment for the predecessor product that, in the case of a contrast agent, is packaged into the payment forthe contrast-enhanced procedure in which the contrast agent is used. For CY 2010, when a contrast agentwith pass-through status is billed with a contrast-enhanced procedure assigned to any procedural APC listedin this section’s table on the same date of service, a specific pass-through payment offset determined by theprocedural APC to which the contrast-enhanced procedure is assigned will be applied to payment for thecontrast agent to ensure that duplicate payment is not made for the contrast agent.Effective January 1, 2009, contrast agent HCPCS code C9246 (Injection, gadoxetate disodium, per ml) wasgranted pass-through status under the OPPS and was assigned status indicator “G.” As the pass-throughoffset methodology was not in place for contrast agents in CY 2009, payments for HCPCS code C9246 werenot reduced by the corresponding contrast-enhanced procedure’s portion of its APC payment associatedwith “policy-packaged” drugs (offset amount).For CY 2010, HCPCS code C9246 is being replaced with HCPCS code A9581 (Injection, gadoxetatedisodium, 1 ml) and HCPCS code A9581 will continue on pass-through status for CY 2010. In addition,HCPCS code A9583 (Injection, gadofosveset trisodium, 1 ml) describes a contrast agent that has beengranted pass-through status beginning January 1, 2010. Both HCPCS codes A9581 and A9583 will beassigned status indicator “G” and will be subject to the payment offset methodology for contrast agents.Home Health & Hospice February 1, 2010 32Medicare A Newsline Vol. 17, No. 5
- Page 7: use and while they may serve other
- Page 10: The following additional billing re
- Page 13 and 14: APPENDIX 1TABULAR PRESENTATION OF T
- Page 15 and 16: News from CMS for Home Health Provi
- Page 17 and 18: Provider Types AffectedHome health
- Page 19 and 20: • For CY 2010, Medicare is modify
- Page 21 and 22: procedures since 2005, continue to
- Page 23 and 24: methodology. The services are paid
- Page 25 and 26: NOTE: When billing for stranded sou
- Page 27 and 28: c. Other Changes to CY 2010 HCPCS C
- Page 29 and 30: d. Drugs and Biologicals with Payme
- Page 31: separately payable status under the
- Page 35 and 36: CY 2010 APC CY 2010 APC Title0375 A
- Page 37 and 38: m. Pricer will update the payment r
- Page 39 and 40: If you have questions regarding thi
- Page 41 and 42: When recording any visit or social
- Page 43 and 44: Submit MSP Claims/Adjustments via:W
- Page 45 and 46: Example 1: ABC Home Health is admit
- Page 47 and 48: Before You Call Cahaba, Read This!F
- Page 49 and 50: Reason for CallClaim needingcorrect
- Page 51 and 52: Availability of the Provider Contac
- Page 53 and 54: Cahaba GBA Learning Corner‣ “Ho
- Page 55 and 56: Cahaba GBA Learning Corner‣ “OA
CY <strong>2010</strong> APCCY <strong>2010</strong> APC Title0395 GI Tract Imaging0396 Bone Imaging0397 Vascular Imaging0398 Level I Cardiac Imaging0400 Hematopoietic Imaging0401 Level I Pulmonary Imaging0402 Level II Nervous System Imaging0403 Level I Nervous System Imaging0404 Renal and Genitourinary Studies0406 Level I Tumor/Infection Imaging0408 Level III Tumor/Infection Imaging0414 Level II Tumor/Infection Imagingl. Introduction of Payment Offset for Pass-Through Contrast AgentsAs discussed in the CY <strong>2010</strong> OPPS/ASC final rule with comment period, effective for pass-through contrastagents furnished on and after January 1, <strong>2010</strong>, when a contrast-enhanced procedure that is assigned to aprocedural APC with a “policy-packaged” drug amount greater than $20 (that is not an APC containingnuclear medicine procedures) is billed on the same claim with a pass-through contrast agent on the samedate of service, CMS will reduce the amount of payment for the contrast agent by the correspondingcontrast-enhanced procedure’s portion of its APC payment associated with “policy-packaged” drugs (offsetamount) so no duplicate contrast agent payment is made.CY <strong>2010</strong> procedural APCs for which CMS expects a contrast agent payment offset could be applicable inthe case of a pass-through contrast agent are identified in the table below this section. Pass-throughpayment for a contrast agent is the difference between the payment for the pass-through product and thepayment for the predecessor product that, in the case of a contrast agent, is packaged into the payment forthe contrast-enhanced procedure in which the contrast agent is used. For CY <strong>2010</strong>, when a contrast agentwith pass-through status is billed with a contrast-enhanced procedure assigned to any procedural APC listedin this section’s table on the same date of service, a specific pass-through payment offset determined by theprocedural APC to which the contrast-enhanced procedure is assigned will be applied to payment for thecontrast agent to ensure that duplicate payment is not made for the contrast agent.Effective January 1, 2009, contrast agent HCPCS code C9246 (Injection, gadoxetate disodium, per ml) wasgranted pass-through status under the OPPS and was assigned status indicator “G.” As the pass-throughoffset methodology was not in place for contrast agents in CY 2009, payments for HCPCS code C9246 werenot reduced by the corresponding contrast-enhanced procedure’s portion of its APC payment associatedwith “policy-packaged” drugs (offset amount).For CY <strong>2010</strong>, HCPCS code C9246 is being replaced with HCPCS code A9581 (Injection, gadoxetatedisodium, 1 ml) and HCPCS code A9581 will continue on pass-through status for CY <strong>2010</strong>. In addition,HCPCS code A9583 (Injection, gadofosveset trisodium, 1 ml) describes a contrast agent that has beengranted pass-through status beginning January 1, <strong>2010</strong>. Both HCPCS codes A9581 and A9583 will beassigned status indicator “G” and will be subject to the payment offset methodology for contrast agents.<strong>Home</strong> <strong>Health</strong> & <strong>Hospice</strong> <strong>February</strong> 1, <strong>2010</strong> 32<strong>Medicare</strong> A Newsline Vol. 17, No. 5