Focused on the patient - Baylor Health Care System
Focused on the patient - Baylor Health Care System
Focused on the patient - Baylor Health Care System
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
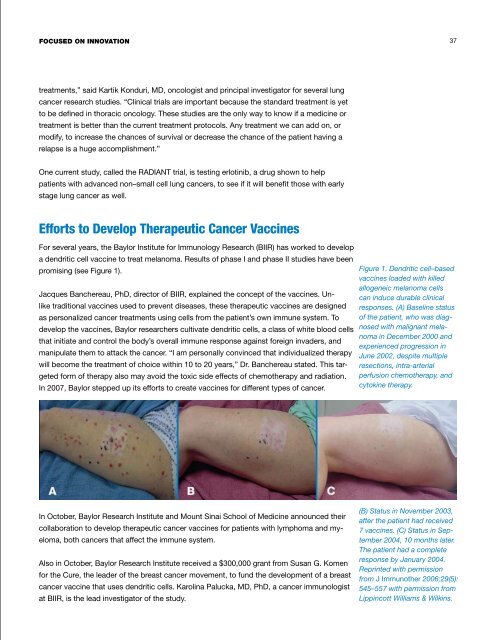
<str<strong>on</strong>g>Focused</str<strong>on</strong>g> <strong>on</strong> innovati<strong>on</strong> 37treatments,” said Kartik K<strong>on</strong>duri, MD, <strong>on</strong>cologist and principal investigator for several lungcancer research studies. “Clinical trials are important because <strong>the</strong> standard treatment is yetto be defined in thoracic <strong>on</strong>cology. These studies are <strong>the</strong> <strong>on</strong>ly way to know if a medicine ortreatment is better than <strong>the</strong> current treatment protocols. Any treatment we can add <strong>on</strong>, ormodify, to increase <strong>the</strong> chances of survival or decrease <strong>the</strong> chance of <strong>the</strong> <strong>patient</strong> having arelapse is a huge accomplishment.”One current study, called <strong>the</strong> RADIANT trial, is testing erlotinib, a drug shown to help<strong>patient</strong>s with advanced n<strong>on</strong>–small cell lung cancers, to see if it will benefit those with earlystage lung cancer as well.Efforts to Develop Therapeutic Cancer VaccinesFor several years, <strong>the</strong> <strong>Baylor</strong> Institute for Immunology Research (BIIR) has worked to developa dendritic cell vaccine to treat melanoma. Results of phase I and phase II studies have beenpromising (see Figure 1).Jacques Banchereau, PhD, director of BIIR, explained <strong>the</strong> c<strong>on</strong>cept of <strong>the</strong> vaccines. Unliketraditi<strong>on</strong>al vaccines used to prevent diseases, <strong>the</strong>se <strong>the</strong>rapeutic vaccines are designedas pers<strong>on</strong>alized cancer treatments using cells from <strong>the</strong> <strong>patient</strong>’s own immune system. Todevelop <strong>the</strong> vaccines, <strong>Baylor</strong> researchers cultivate dendritic cells, a class of white blood cellsthat initiate and c<strong>on</strong>trol <strong>the</strong> body’s overall immune resp<strong>on</strong>se against foreign invaders, andmanipulate <strong>the</strong>m to attack <strong>the</strong> cancer. “I am pers<strong>on</strong>ally c<strong>on</strong>vinced that individualized <strong>the</strong>rapywill become <strong>the</strong> treatment of choice within 10 to 20 years,” Dr. Banchereau stated. This targetedform of <strong>the</strong>rapy also may avoid <strong>the</strong> toxic side effects of chemo<strong>the</strong>rapy and radiati<strong>on</strong>.In 2007, <strong>Baylor</strong> stepped up its efforts to create vaccines for different types of cancer.Figure 1. Dendritic cell–basedvaccines loaded with killedallogeneic melanoma cellscan induce durable clinicalresp<strong>on</strong>ses. (A) Baseline statusof <strong>the</strong> <strong>patient</strong>, who was diagnosedwith malignant melanomain December 2000 andexperienced progressi<strong>on</strong> inJune 2002, despite multipleresecti<strong>on</strong>s, intra-arterialperfusi<strong>on</strong> chemo<strong>the</strong>rapy, andcytokine <strong>the</strong>rapy.In October, <strong>Baylor</strong> Research Institute and Mount Sinai School of Medicine announced <strong>the</strong>ircollaborati<strong>on</strong> to develop <strong>the</strong>rapeutic cancer vaccines for <strong>patient</strong>s with lymphoma and myeloma,both cancers that affect <strong>the</strong> immune system.Also in October, <strong>Baylor</strong> Research Institute received a $300,000 grant from Susan G. Komenfor <strong>the</strong> Cure, <strong>the</strong> leader of <strong>the</strong> breast cancer movement, to fund <strong>the</strong> development of a breastcancer vaccine that uses dendritic cells. Karolina Palucka, MD, PhD, a cancer immunologistat BIIR, is <strong>the</strong> lead investigator of <strong>the</strong> study.(B) Status in November 2003,after <strong>the</strong> <strong>patient</strong> had received7 vaccines. (C) Status in September2004, 10 m<strong>on</strong>ths later.The <strong>patient</strong> had a completeresp<strong>on</strong>se by January 2004.Reprinted with permissi<strong>on</strong>from J Immuno<strong>the</strong>r 2006;29(5):545–557 with permissi<strong>on</strong> fromLippincott Williams & Wilkins.