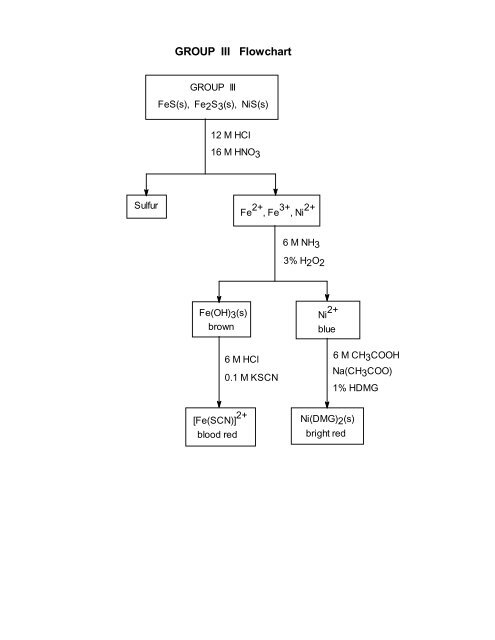
GROUP III Flowchart
GROUP III Flowchart
GROUP III Flowchart
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>GROUP</strong> <strong>III</strong> <strong>Flowchart</strong><strong>GROUP</strong> <strong>III</strong>FeS(s), Fe2S3(s), NiS(s)12 M HCl16 M HNO3SulfurFe 2+ , Fe 3+ , Ni 2+ Ni 2+6 M NH33% H2O2Fe(OH)3(s)brownblue6 M HCl0.1 M KSCN6 M CH3COOHNa(CH3COO)1% HDMG[Fe(SCN)] 2+blood redNi(DMG)2(s)bright red
Cation Qualitative Analysis__________________________________________________________________PART <strong>III</strong>: Cation Group <strong>III</strong>Separation of Cation Group <strong>III</strong>40. To 4 mL of the solution saved from step 24, add 0.5 mL of 6 M HCl.41. Add 6 M NH 3 dropwise, with stirring, until the resulting suspension has a pH = 9(check with pH paper).42. Add 15 drops of 1 M thioacetamide and heat the mixture in the hot water bath in thehood for 10 minutes.43. Centrifuge and decant, saving both the supernatant and precipitate. Label and savethe supernatent for the analysis of Group IV (step 64). Save the precipitate for CationGroup <strong>III</strong> analysis in the next step.Cation Group <strong>III</strong> AnalysisDissolution of Cation Group <strong>III</strong>44. Add 1 mL of deionized water to the precipitate._______________________________________________________________________CAUTION The concentrated HCl used in step 45 is highly corrosive, and can cause severe damage toeyes, skin, and clothing. In case of skin contact, immediately wash the area with large amounts of water.45. Add 0.5 mL of 12 M HCl.46. Boil gently for 3 minutes in the hot water bath in the hood._______________________________________________________________________CAUTION The concentrated HNO 3 used in step 47 is highly corrosive, and can cause severe damage toeyes, skin, and clothing. In case of skin contact, immediately wash the area with large amounts of water.47. If an undissolved residue remains, add 3 to 4 drops of 16 M HNO 3 and boil gentlyuntil a clear solution is formed.48. Dilute to 2 mL with deionized water.49. Discard any precipitated sulfur.50. Save the solution which now contains Fe 3+ and Ni 2+ for the next step.Separation of the Fe 3+ and Ni 2+ Cations51. To the solution (from step 50) add 6 M NH 3 dropwise, with stirring, until the solutionis just alkaline. Check with litmus paper.52. Add 1 additional mL of 6 M NH 3 .53. Add 10 drops of 3% hydrogen peroxide (H 2 O 2 ), with stirring.54. Heat the solution in the hot water bath for 1-2 minutes to coagulate any Fe(OH) 3precipitate.55. Let the solution stand for 5 minutes.56. Centrifuge and decant saving both the supernatent and precipitate. Save thesupernatant to test for Ni 2+ (step 60). Save the precipitate to test for Fe 3+ in the next step.
Cation Qualitative Analysis__________________________________________________________________Confirmation of the Fe 3+ Cation57. To dissolve the precipitate from step 56, add 1 mL of 6 M HCl, with stirring.58. Add 2 drops of 0.1 M KSCN.59. Record your observations. A blood-red solution produced by the thiocyanatoiron(<strong>III</strong>)complex ion, [Fe(SCN)] 2+ , indicates the presence of the iron(<strong>III</strong>) cation (Fe 3+ ).Confirmation of the Ni 2+ Cation60. To the supernatant from step 56 add 6 M CH 3 COOH dropwise, with stirring, until thesolution is acidic. Check with litmus paper.61. Add solid Na(CH 3 COO) in small portions, with stirring, until the solution issaturated.62. Add 5 - 10 drops of 1% dimethylglyoxime solution.63. Record your observations. The formation of a bright-red precipitate indicates thepresence of the nickel(II) cation (Ni 2+ ).
Cation Qualitative AnalysisGroup <strong>III</strong>Name____________________CHE 102L Section_____Date _______Unknown # _____Cation Report:(circle one)Fe 2+/3+ Present Absent (15 pts)Ni 2+ Present Absent (15 pts)GRADE 80 65 50 0