The Refrigeration Load - HVAC and Refrigeration Information Links
The Refrigeration Load - HVAC and Refrigeration Information Links The Refrigeration Load - HVAC and Refrigeration Information Links
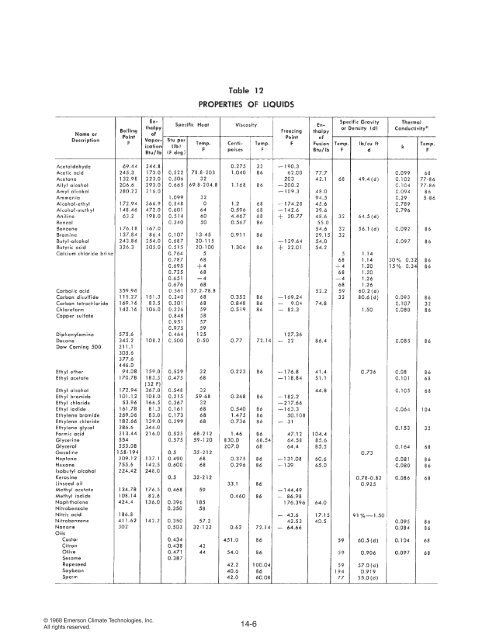
© 1968 Emerson Climate Technologies, Inc.All rights reserved. 14-6
SENSIBLE HEAT ABOVE FREEZINGMost products are at a higher temperature than the storagetemperature when placed in a refrigerator. Sincemany foods have a high percentage of water content,their reaction to a loss of heat is quite different aboveand below the freezing point. Above the freezing point,the water exists in liquid form, while below the freezingpoint, the water has changed its state to ice.As mentioned previously, the specific heat of a productis defined as the BTUs required to raise the temperatureof one pound of the substance 1°F. The specific heatsof various commodities are listed in Tables 10, 11, and12. Note that in Table 10 the specific heat of the productabove freezing is different than the specific heat belowfreezing, and the freezing point (listed in the first column)varies, but in practically all cases is below 32°F.The heat to be removed from a product to reduce itstemperature above freezing may be calculated as follows:Q = W x c x (T 1- T 2)Q = BTU to be removedW = Weight of the product in poundsc = Specific heat above freezingT 1= Initial temperature, °F.T 2= Initial temperature, °F. (freezing or above)For example, the heat to be removed in order to cool1,000 pounds of veal (whose freezing point is 29°F.)from 42°F. to 29°F. can be calculated as follows:Q = W x c x (T 1- T 2)= 1000 pounds x .71 specific heat x (42-29)= 1000 x .71 x 13= 9,230 BTU14-7© 1968 Emerson Climate Technologies, Inc.All rights reserved.
- Page 2 and 3: FOREWORDThe practice of refrigerati
- Page 4 and 5: INDEX OF TABLESTable 4 Typical Heat
- Page 7 and 8: VALUES OF THERMAL CONDUCTIVITY FORB
- Page 9 and 10: 12-5© 1968 Emerson Climate Technol
- Page 11 and 12: 12-7© 1968 Emerson Climate Technol
- Page 13: QUICK CALCULATION TABLE FORWALK-IN
- Page 16 and 17: © 1968 Emerson Climate Technologie
- Page 18 and 19: © 1968 Emerson Climate Technologie
- Page 20 and 21: © 1968 Emerson Climate Technologie
- Page 24 and 25: LATENT HEAT OF FREEZINGThe latent h
- Page 26 and 27: © 1968 Emerson Climate Technologie
- Page 28 and 29: © 1968 Emerson Climate Technologie
- Page 30 and 31: Section 15SUPPLEMENTARY LOADIn addi
- Page 32 and 33: (A)HEAT TRANSMISSION LOAD(E) REQUIR
- Page 34 and 35: © 1968 Emerson Climate Technologie
- Page 36 and 37: © 1968 Emerson Climate Technologie
- Page 38 and 39: © 1968 Emerson Climate Technologie
- Page 40 and 41: © 1968 Emerson Climate Technologie
- Page 42 and 43: Table 18Table 19© 1968 Emerson Cli
- Page 44: Form No. AE 103 R3 (10/06)Emerson®
© 1968 Emerson Climate Technologies, Inc.All rights reserved. 14-6