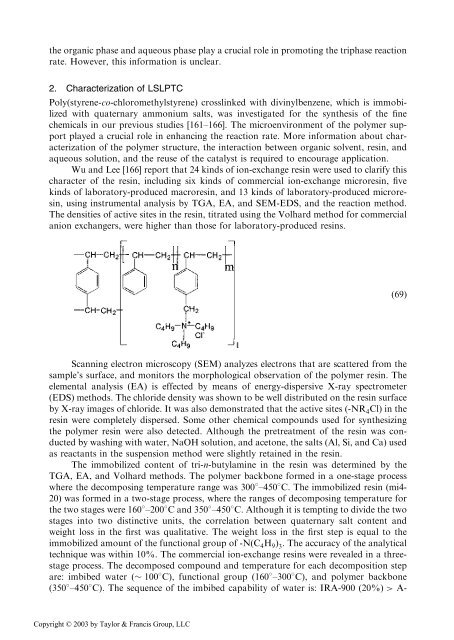
the organic phase <strong>and</strong> aqueous phase play a crucial role in promoting the triphase reactionrate. However, this information is unclear.2. Characterization <strong>of</strong> LSLPTCPoly(styrene-co-chloromethylstyrene) crosslinked with divinylbenzene, which is immobilizedwith quaternary ammonium salts, was investigated for the synthesis <strong>of</strong> the finechemicals in our previous studies [161–166]. The microenvironment <strong>of</strong> the polymer supportplayed a crucial role in enhancing the reaction rate. More information about characterization<strong>of</strong> the polymer structure, the interaction between organic solvent, resin, <strong>and</strong>aqueous solution, <strong>and</strong> the reuse <strong>of</strong> the catalyst is required to encourage application.Wu <strong>and</strong> Lee [166] report that 24 kinds <strong>of</strong> ion-exchange resin were used to clarify thischaracter <strong>of</strong> the resin, including six kinds <strong>of</strong> commercial ion-exchange microresin, fivekinds <strong>of</strong> laboratory-produced macroresin, <strong>and</strong> 13 kinds <strong>of</strong> laboratory-produced microresin,using instrumental analysis by TGA, EA, <strong>and</strong> SEM-EDS, <strong>and</strong> the reaction method.The densities <strong>of</strong> active sites in the resin, titrated using the Volhard method for commercialanion exchangers, were higher than those for laboratory-produced resins.ð69ÞScanning electron microscopy (SEM) analyzes electrons that are scattered from thesample’s surface, <strong>and</strong> monitors the morphological observation <strong>of</strong> the polymer resin. Theelemental analysis (EA) is effected by means <strong>of</strong> energy-dispersive X-ray spectrometer(EDS) methods. The chloride density was shown to be well distributed on the resin surfaceby X-ray images <strong>of</strong> chloride. It was also demonstrated that the active sites (-NR 4 Cl) in theresin were completely dispersed. Some other chemical compounds used for synthesizingthe polymer resin were also detected. Although the pretreatment <strong>of</strong> the resin was conductedby washing with water, NaOH solution, <strong>and</strong> acetone, the salts (Al, Si, <strong>and</strong> Ca) usedas reactants in the suspension method were slightly retained in the resin.The immobilized content <strong>of</strong> tri-n-butylamine in the resin was determined by theTGA, EA, <strong>and</strong> Volhard methods. The polymer backbone formed in a one-stage processwhere the decomposing temperature range was 300 –450 C. The immobilized resin (mi4-20) was formed in a two-stage process, where the ranges <strong>of</strong> decomposing temperature forthe two stages were 160 –200 C <strong>and</strong> 350 –450 C. Although it is tempting to divide the twostages into two distinctive units, the correlation between quaternary salt content <strong>and</strong>weight loss in the first was qualitative. The weight loss in the first step is equal to theimmobilized amount <strong>of</strong> the functional group <strong>of</strong> -NðC 4 H 9 Þ 3 . The accuracy <strong>of</strong> the analyticaltechnique was within 10%. The commercial ion-exchange resins were revealed in a threestageprocess. The decomposed compound <strong>and</strong> temperature for each decomposition stepare: imbibed water ( 100 C), functional group (160 –300 C), <strong>and</strong> polymer backbone(350 –450 C). The sequence <strong>of</strong> the imbibed capability <strong>of</strong> water is: IRA-900 ð20%Þ > A-Copyright © 2003 by Taylor & Francis Group, LLC
26 > Dowex 1 2 > A-27 IRA-410 > IRA-904 > mi4-20 (4%). Most commercial ionexchangeresins are <strong>of</strong> the hydrophilic functional group type.In addition, the immobilized amount <strong>of</strong> the functional group <strong>of</strong> -NðC 4 H 9 Þ 3 in theresin was determined from the mass fraction <strong>of</strong> nitrogen by EA for C, H, <strong>and</strong> N, <strong>and</strong> fromthe chloride ion density titrated by the Volhard method. The sequence <strong>of</strong> determiningmethod for the immobilized content <strong>of</strong> tri-n-butylamine in the resin wasTGA > EA > Volhard. The analyzed result <strong>of</strong> the TGA (or EA) method was based onthe elemental weight, <strong>and</strong> it revealed the real immobilized content. However, the analyzedresult <strong>of</strong> the Volhard method determined the free chloride ion in the solution by theAgNO 3 titration method. The immobilized content <strong>of</strong> tri-n-butylamine in the resin bythe TGA (or EA) method was > 20% larger than that determined by the Volhard method.The immobilized content <strong>of</strong> tri-n-butylamine in the resin by the TGA (or EA) method wasindependent <strong>of</strong> the number <strong>of</strong> cross-linkages, <strong>and</strong> only dependent <strong>of</strong> the number <strong>of</strong> thering substitution.These experimental results demonstrate that tri-n-butylamine could be immobilizedcompletely with the active site on the resin for an immobilizion duration <strong>of</strong> 6 days.However, the immobilized content <strong>of</strong> tri-n-butylamine by the Volhard method was dependenton both the number <strong>of</strong> cross-linkages <strong>and</strong> the number <strong>of</strong> ring substitutions. Theimmobilized contents for the Volhard method are about 50–70% that for TGA (orEA). Since the analyzed results <strong>of</strong> the Volhard method determined the free chlorideions in the solution by the AgNO 3 titration method, the free chloride ion <strong>of</strong> the activesite were only measured at 50–70% <strong>of</strong> the amount <strong>of</strong> immobilized content. The trend <strong>of</strong>the varied content for microresin is larger than that for macroresin. This result indicatesthat the analysis by the Volhard method may be influenced by the diffusion problem, <strong>and</strong>may be because the resin did not swell completely in the aqueous solution. On the otherh<strong>and</strong>, if the resin is used as a TC to react in an actual reaction system, <strong>and</strong> the resin couldnot swell completely to release all free chloride ions, then the reaction environment wouldbe influenced by the mass transfer <strong>of</strong> the reactant.As indicated by Ohtani et al. [32] both organic reactant <strong>and</strong> aqueous reactant existwithin the pores <strong>of</strong> the polymer pellet. The HLB <strong>of</strong> the support structure determines thedistribution <strong>of</strong> the two phases within the catalyst support [167,168]. Therefore, the distribution<strong>of</strong> the organic reactant <strong>and</strong> aqueous reactant within the pores <strong>of</strong> the polymerpellet will directly influence the reaction. The swollen capability <strong>of</strong> the resin is used toestimate the validity <strong>of</strong> the resin. The effect factor <strong>of</strong> the swollen capability <strong>of</strong> the resinincludes the cross-linkage, the number <strong>of</strong> ring substitutions (total exchange capability), theelectronic charge <strong>and</strong> diameter <strong>of</strong> the counterion, the polarity <strong>of</strong> the organic solvent, thecomposition <strong>of</strong> the functional group, the chemical bonding type between both exchangeions, <strong>and</strong> the electrolyte concentration in the aqueous solution.Wu <strong>and</strong> Lee [166] <strong>and</strong> Tang [169] reported the amount <strong>of</strong> imbibed solvent, volumeratio, <strong>and</strong> porosity <strong>of</strong> 12 kinds <strong>of</strong> ion-exchange resin for seven kinds <strong>of</strong> solvents (dichloromethane,chlor<strong>of</strong>orm, 1,2-dichloroethane, benzene, toluene, chlorobenzene, <strong>and</strong> water)when 1 g <strong>of</strong> the resin was placed in 25 mL <strong>of</strong> the pure solvent. The experimental results forthe commercial ion-exchange resin were as follows:1. The amounts <strong>of</strong> the imbibed solvent for the aromatic solvents (benzene,toluene, <strong>and</strong> chlorobenzene) were larger than those for halide aliphatic solvents(dichloromethane, chlor<strong>of</strong>orm, <strong>and</strong> 1,2-dichloroethane) since the resin was <strong>of</strong>the styrene type; the sequence <strong>of</strong> the imbibed amount for the aromatic solventswas benzene > toluene > chlorobenzene.Copyright © 2003 by Taylor & Francis Group, LLC