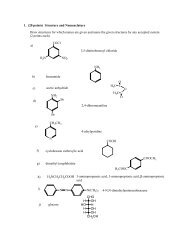
S-1 EXPERIMENT S: THE NERNST EQUATION AND ITS ...
S-1 EXPERIMENT S: THE NERNST EQUATION AND ITS ...
S-1 EXPERIMENT S: THE NERNST EQUATION AND ITS ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(c) Calculate K sp . ( 0.5 mark )S-3
S-4REPORT: <strong>EXPERIMENT</strong> S - APPLICATIONS OF <strong>THE</strong> <strong>NERNST</strong> <strong>EQUATION</strong>Name ______________________________Lab Period _________ Lab Room ________TA ________________________________Show your work - answers without supporting work will get zero marks. Units and the correctnumber of significant figures are required. Graphs must conform to the Guidelines on Graphingon p. 33 in the front part of this Manual.Part I. The Effect of Concentration on Electrode PotentialsA. Transfer Ecell values from your Observations Sheet to the following Table:[Ag + ] log 1Measured cell[Ag + ] potential (volts)set 1set 2set 3set 4set 5B.1. Using your results from the measured cell potentials in the above Table, draw a graph of E cellversus log(1/[Ag + ]) for Part I. Draw a "best-fit" straight line through your results (3marks). Attach this graph to your Report.2. Show on your graph, your calculationa. for the slope of the best-fit (experimental) straight line through the measured points.(1 mark)b. starting from Equation (9), of the theoretical slope of the line. ( 1 mark ).Part II E° and Equilibrium ConstantA.1. Plot your results for Part II as E cell versus log(1/[Ag + ]) . This graph will not look like thegraph for Part I. Draw a "best-fit" straight line through your results and extrapolate (dashedline) to the x-axis where E cell = 0. Attach this graph to your Report. (3 marks)2. Indicate on your graph, the value of log Q cathode where E cell = 0. Use this value to calculate( show your work on the graph ) the corresponding [Ag + ] at this point. (1 mark).
S-7OBSERVATIONS SHEET - <strong>EXPERIMENT</strong> SThe Nernst Equation and Its ApplicationsPart I: The Effect of Concentration on Electrode PotentialsThe zero point value was out by _____________ volts.[Ag + ]in test half-cell E cell + electrode12345Part II: E° and Equilibrium Constant12345[Ag + ]in test half-cell E cell + electrodePart III: The Formation Constant of a Complex IonObservations on addition of 0.05 mL (1 drop) S 2 O 32- to test tube 1 _______________________________________________________________________________________Cell potential after addition of 0.05 mL (1 drop) of S 2 O 3 2- : __________________Which electrode was the cathode __________________Observations on addition of 2.0 mL S 2 O 32- to test tube 2: ____________________________________________________________________________________________________Cell potential after addition of 2.0 mL of S 2 O 3 2- : __________________Which electrode was the cathode ______________________________________________ ____________________________ _________Signature of Lab Instructor Name of Student Lab Period
S-8OBSERVATIONS SHEET - <strong>EXPERIMENT</strong> SThe Nernst Equation and Its ApplicationsPart I: The Effect of Concentration on Electrode PotentialsThe zero point value was out by _____________ volts.[Ag + ]in test half-cell E cell + electrode12345Part II: E° and Equilibrium Constant12345[Ag + ]in test half-cell E cell + electrodePart III: The Formation Constant of a Complex IonObservations on addition of 0.05 mL (1 drop) S 2 O 32- to test tube 1 _______________________________________________________________________________________Cell potential after addition of 0.05 mL (1 drop) of S 2 O 3 2- : __________________Which electrode was the cathode __________________Observations on addition of 2.0 mL S 2 O 32- to test tube 2: ____________________________________________________________________________________________________Cell potential after addition of 2.0 mL of S 2 O 3 2- : __________________Which electrode was the cathode ______________________________________________ ____________________________ _________Signature of Lab Instructor Name of Student Lab Period