Chem 232 Lecture 12 - UIC Department of Chemistry - University of ...
Chem 232 Lecture 12 - UIC Department of Chemistry - University of ... Chem 232 Lecture 12 - UIC Department of Chemistry - University of ...
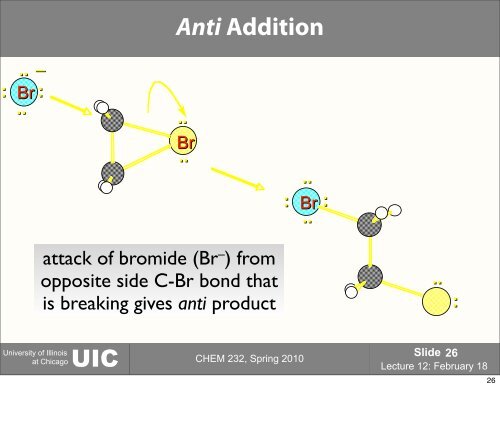
Anti Addition..–: Br:....Br..:..Br..:attack of bromide (Br – ) fromopposite side C-Br bond thatis breaking gives anti product..:University of IllinoisUICat Chicago CHEM 232, Spring 2010Slide 26Lecture 12: February 1826
Hyperconjugation (σ C-H →π C=C )Increases Rate of HalogenationHC C C HHσ➞∏donationalkeneH HH HH 3 C HH HH 3 C HH 3 C HH 3 C CH 3H 3 C CH 3relative rate (krel)1615400920,000• more alkyl groups attached to double bond ➡• more σ➞π orbital donation/overlap ➡• double bond has more electron density ➡• double bond is a stronger nucleophile (Lewis base) ➡• reacts faster with electrophiles like Br-BrUniversity of IllinoisUICat Chicago CHEM 232, Spring 2010Slide 27Lecture 12: February 1827
- Page 1 and 2: CHEM 232Organic Chemistry IUniversi
- Page 3 and 4: CHEM 232Organic Chemistry IUniversi
- Page 5 and 6: Boranes - Structure & ElectronicsHH
- Page 8 and 9: Stoichiometry of Hydroboration6 xB
- Page 10 and 11: Oxidation StephydroborationB 2 H 6d
- Page 12 and 13: Regioselectivity DeterminedDuring H
- Page 14 and 15: Regioselectivity - 1st RationaleSte
- Page 16 and 17: Self Test QuestionWhat is the produ
- Page 18 and 19: Alkene-Halogen AdditionC C + X Yadd
- Page 20 and 21: Halogenation is Stereospecific• a
- Page 22 and 23: Stereospecific ReactionsStereospeci
- Page 24 and 25: Halogen Addition DoesNot Involve Fr
- Page 28 and 29: Halogen Addition Modification:Aqueo
- Page 30 and 31: CHEM 232Organic Chemistry IUniversi
- Page 32 and 33: Epoxides are Preparedvia Alkene Epo
- Page 34 and 35: Alkenes are Cleaved via Ozonolysiso
- Page 36 and 37: CHEM 232Organic Chemistry IUniversi
- Page 38 and 39: Synthetic RouteHow could you prepar
- Page 40 and 41: Self Test QuestionDevise a retrosyn
- Page 42 and 43: Self Test QuestionWhich starting ma
- Page 44: Quiz & Exam AveragesQuiz 1 = 61%Qui
Anti Addition..–: Br:....Br..:..Br..:attack <strong>of</strong> bromide (Br – ) fromopposite side C-Br bond thatis breaking gives anti product..:<strong>University</strong> <strong>of</strong> Illinois<strong>UIC</strong>at Chicago CHEM <strong>232</strong>, Spring 2010Slide 26<strong>Lecture</strong> <strong>12</strong>: February 1826