Nomenclature Flow Chart - Chandler-Gilbert Community College
Nomenclature Flow Chart - Chandler-Gilbert Community College
Nomenclature Flow Chart - Chandler-Gilbert Community College
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
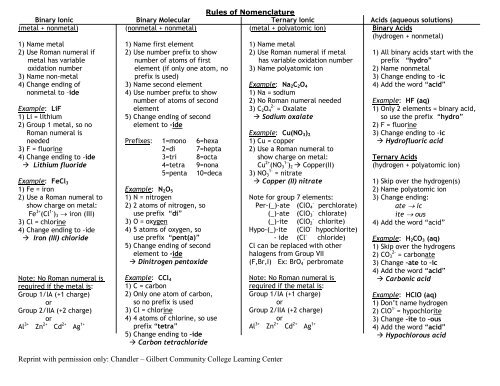
Rules of <strong>Nomenclature</strong>Binary Ionic Binary Molecular Ternary Ionic Acids (aqueous solutions)(metal + nonmetal)(nonmetal + nonmetal)(metal + polyatomic ion)Binary AcidsAl 3+ Zn 2+ Cd 2+ Ag 1+ prefix “tetra”Al 3+ Zn 2+ Cd 2+ Ag 1+ 4) Add the word “acid”(hydrogen + nonmetal)1) Name metal2) Use Roman numeral ifmetal has variableoxidation number3) Name non-metal4) Change ending ofnonmetal to –ideExample: LiF1) Li = lithium2) Group 1 metal, so noRoman numeral is1) Name first element2) Use number prefix to shownumber of atoms of firstelement (if only one atom, noprefix is used)3) Name second element4) Use number prefix to shownumber of atoms of secondelement5) Change ending of secondelement to –ide1) Name metal2) Use Roman numeral if metalhas variable oxidation number3) Name polyatomic ionExample: Na 2 C 2 O 41) Na = sodium2) No Roman numeral needed3) C 2 O 2- 4 = Oxalate Sodium oxalateExample: Cu(NO 3 ) 21) All binary acids start with theprefix “hydro”2) Name nonmetal3) Change ending to -ic4) Add the word “acid”Example: HF (aq)1) Only 2 elements = binary acid,so use the prefix “hydro”2) F = fluorine3) Change ending to -icneededPrefixes: 1=mono 6=hexa 1) Cu = copper Hydrofluoric acid3) F = fluorine2=di 7=hepta 2) Use a Roman numeral to4) Change ending to -ide3=tri 8=octashow charge on metal:Ternary Acids Lithium fluoride4=tetra 9=nonaCu 2+ (NO 1- 3 ) 2 Copper(II)(hydrogen + polyatomic ion)5=penta 10=deca 3) NO 1- 3 = nitrateExample: FeCl 3 Copper (II) nitrate1) Skip over the hydrogen(s)1) Fe = iron2) Use a Roman numeral toExample: N 2 O 51) N = nitrogenNote for group 7 elements:2) Name polyatomic ion3) Change ending:show charge on metal: 2) 2 atoms of nitrogen, so-Per-(_)-ate (ClO 4 perchlorate)ate icFe 3+ (Cl 1- -) 3 iron (III)use prefix “di”(_)-ate (ClO 3 chlorate)ite ous-3) Cl = chlorine3) O = oxygen(_)-ite (ClO 2 chlorite) 4) Add the word “acid”4) Change ending to -ide 4) 5 atoms of oxygen, soHypo-(_)-ite (ClO - hypochlorite) Iron (III) chlorideuse prefix “pent(a)”- ide (Cl - chloride) Example: H 2 CO 3 (aq)5) Change ending of secondCl can be replaced with other 1) Skip over the hydrogenselement to -idehalogens from Group VII2) CO 2- 3 = carbonate Dinitrogen pentoxide(F,Br,I) Ex: BrO - 4 perbromate 3) Change –ate to –ic4) Add the word “acid”Note: No Roman numeral isrequired if the metal is:Group 1/IA (+1 charge)orGroup 2/IIA (+2 charge)orExample: CCl 41) C = carbon2) Only one atom of carbon,so no prefix is used3) Cl = chlorine4) 4 atoms of chlorine, so useNote: No Roman numeral isrequired if the metal is:Group 1/IA (+1 charge)orGroup 2/IIA (+2 charge)or Carbonic acidExample: HClO (aq)1) Don’t name hydrogen2) ClO 1- = hypochlorite3) Change –ite to –ous5) Change ending to –ide Carbon tetrachloride Hypochlorous acidReprint with permission only: <strong>Chandler</strong> – <strong>Gilbert</strong> <strong>Community</strong> <strong>College</strong> Learning Center
<strong>Nomenclature</strong> <strong>Flow</strong> <strong>Chart</strong>YESHow manyelementsare in thecompound?Only 2Morethan 2Binary Ionic (Category I)Metal + NonmetalTernary Ionic (Category II)Metal + Polyatomic IonIs the first elementa metal?NOIs hydrogen thefirst element, and isthe compound inaqueous solution?NOBinary Molecular/Covalent(Category III)Nonmetal + NonmetalYESBinary Acid (Category IV)Hydrogen + NonmetalHow manyelementsare in thecompound?Only 2Morethan 2Ternary Acid (Category V)Hydrogen + Polyatomic IonReprint with permission only: <strong>Chandler</strong> – <strong>Gilbert</strong> <strong>Community</strong> <strong>College</strong> Learning Center