Chapter 6 Periodic Table Lecture Notes.pdf
Chapter 6 Periodic Table Lecture Notes.pdf
Chapter 6 Periodic Table Lecture Notes.pdf
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The <strong>Periodic</strong> <strong>Table</strong> and <strong>Periodic</strong> LawSection 6.1 Development of theModern <strong>Periodic</strong> <strong>Table</strong>Section 6.2 Classification of theElementsSection 6.3 <strong>Periodic</strong> TrendsClick a hyperlink or folder tab to viewthe corresponding slides.Exit
Section 6.1 Development of the Modern<strong>Periodic</strong> <strong>Table</strong>• Trace the development of the periodic table.• Identify key features of the periodic table.atomic number: the number of protons in an atomThe periodic table evolved overtime as scientists discovered moreuseful ways to compare andorganize the elements.
Section 6.1 Development of the Modern<strong>Periodic</strong> <strong>Table</strong> (cont.)periodic lawgroupperiodrepresentative elementstransition elementsmetalalkali metalsalkaline earth metalstransition metalinner transition metallanthanide seriesactinide seriesnonmetalshalogennoble gasmetalloid
Development of the <strong>Periodic</strong> <strong>Table</strong>• In the 1700s, Lavoisier compiled a list of allthe known elements of the time.
Development of the <strong>Periodic</strong> <strong>Table</strong> (cont.)• The 1800s brought large amounts ofinformation and scientists needed a way toorganize knowledge about elements.• John Newlands proposed an arrangementwhere elements were ordered by increasingatomic mass.
Development of the <strong>Periodic</strong> <strong>Table</strong> (cont.)• Newlands noticedwhen the elementswere arranged byincreasing atomicmass, theirproperties repeatedevery eighthelement.
Development of the <strong>Periodic</strong> <strong>Table</strong> (cont.)• Meyer and Mendeleev both demonstrateda connection between atomic mass andelemental properties.• Moseley rearranged the table by increasingatomic number, and resulted in a clearperiodic pattern.• <strong>Periodic</strong> repetition of chemical and physicalproperties of the elements when they arearranged by increasing atomic number iscalled periodic law.
Development of the <strong>Periodic</strong> <strong>Table</strong> (cont.)
The Modern <strong>Periodic</strong> <strong>Table</strong> (cont.)• Columns of elements are called groups.• Rows of elements are called periods.• Elements in groups 1,2, and 13-18 possess awide variety of chemical and physicalproperties and are called the representativeelements.• Elements in groups 3-12 are known as thetransition metals.
Periods• Each row is a period• The number of elements per period varies due to theway the orbitals increase from NRG level to NRG level
Groups• Each column in the periodictable is called a group• Elements within a grouphave similar properties• Elements in a group havesimilar electronconfigurations• The electron configurationof an element determinesits chemical properties
Classes of Elements• 3 ways to classify elements– States at room temperature: solid, liquid, gas– Naturally occurring or not naturally occurring(atomic #93 and higher do not occur naturally)– Categories based on their general properties• Metals – located on the left in the periodic table• Non metals – located on the right• Metalloids – in-between
Metals• Most on the periodic table are classified as metals• Properties– Good conductors– Except mercury, they are solid at room temp– Malleable– DuctileGroups 3-12 are transition metalscan form compounds with distinctive colors
Non Metals• Properties onnonmetals– Opposite of metals– Poor conductors– Many are gases atroom temp– The nonmetalsthat are solid atroom temp arevery brittle
Metalloids• Properties of metalloids– Between metals and nonmetals– A metalloid’s ability to conduct electric currentvaries depending on temperature
The Modern <strong>Periodic</strong> <strong>Table</strong> (cont.)• Elements are classified as metals,non-metals, and metalloids.• Metals are elements that are generally shinywhen smooth and clean, solid at roomtemperature, and good conductors of heatand electricity.• Alkali metals are all the elements in group 1except hydrogen, and are very reactive.• Alkaline earth metals are in group 2, andare also highly reactive.
The Modern <strong>Periodic</strong> <strong>Table</strong> (cont.)• The transition elements are divided intotransition metals and inner transitionmetals.• The two sets of inner transition metals arecalled the lanthanide series and actinideseries and are located at the bottom of theperiodic table.
The Modern <strong>Periodic</strong> <strong>Table</strong> (cont.)• Group 17 is composed of highly reactiveelements called halogens.• Group 18 gases are extremely unreactive andcommonly called noble gases.
The Modern <strong>Periodic</strong> <strong>Table</strong> (cont.)• Metalloids have physical and chemicalproperties of both metals and non-metals,such as silicon and germanium.
The Modern <strong>Periodic</strong> <strong>Table</strong> (cont.)
Section 6.1 AssessmentWhat is a row of elements on the periodictable called?A. octaveB. periodC. groupD. transitionABA. AB. BC. C0% 0% 0% 0%D. DCD
Section 6.1 AssessmentWhat is silicon an example of?A. metalB. non-metalC. inner transition metalD. metalloidABA. AB. BC. C0% 0% 0% 0%D. DCD
Section 6.2 Classification of the Elements• Explain why elements inthe same group havesimilar properties.• Identify the four blocksof the periodic tablebased on their electronconfiguration.valence electron:electron in an atom'soutermost orbitals;determines the chemicalproperties of an atomElements are organized into differentblocks in the periodic table accordingto their electron configurations.
Organizing the Elements by ElectronConfiguration• Recall electrons in the highest principalenergy level are called valence electrons.• All group 1 elements have one valenceelectron.
Organizing the Elements by ElectronConfiguration (cont.)• The energy level of an element’s valenceelectrons indicates the period on theperiodic table in which it is found.• The number of valence electrons forelements in groups 13-18 is ten less thantheir group number.
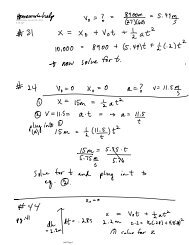
Organizing the Elements by ElectronConfiguration (cont.)
The s-, p-, d-, and f-Block Elements• The shape of the periodic table becomesclear if it is divided into blocks representingthe atom’s energy sublevel being filled withvalence electrons.
The s-, p-, d-, and f-Block Elements (cont.)• s-block elements consist of group 1 and 2,and the element helium.• Group 1 elements have a partially filled sorbital with one electron.• Group 2 elements have a completely filled sorbital with two electrons.
The s-, p-, d-, and f-Block Elements (cont.)• After the s-orbital is filled, valenceelectrons occupy the p-orbital.• Groups 13-18 contain elements withcompletely or partially filled p orbitals.
The s-, p-, d-, and f-Block Elements (cont.)• The d-block contains the transition metalsand is the largest block.• There are exceptions, but d-block elementsusually have filled outermost s orbital, andfilled or partially filled d orbital.• The five d orbitals can hold 10 electrons, sothe d-block spans ten groups on the periodictable.
The s-, p-, d-, and f-Block Elements (cont.)• The f-block contains the inner transitionmetals.• f-block elements have filled or partially filledoutermost s orbitals and filled or partially filled4f and 5f orbitals.• The 7 f orbitals hold 14 electrons, and theinner transition metals span 14 groups.
Section 6.2 AssessmentWhich of the following is NOT one of theelemental blocks of the periodic table?A. s-blockB. d-blockC. g-blockD. f-blockABA. AB. BC. C0% 0% 0% 0%D. DCD
Section 6.2 AssessmentWhich block spans 14 elemental groups?A. s-blockB. p-blockC. f-blockD. g-blockABA. AB. BC. C0% 0% 0% 0%D. DCD
Section 6.3 <strong>Periodic</strong> Trends• Compare period andgroup trends of severalproperties.• Relate period and grouptrends in atomic radii toelectron configuration.principal energy level:the major energy level ofan atomionionization energyoctet ruleelectronegativityTrends among elements in the periodictable include their size and their abilityto lose or attract electrons
• Because of the way the periodic table isorganized today, we can extrapolate a lotof information about an element by lookingat its location on the table– Atomic radii– Ionization energy– Metallic Character– Non-metallic Character– Electronegativity
Atomic Radii• Measure of the size of an element’s atoms• Distance from the nucleus to the surroundingouter edge of the cloud of electrons• Across a period from left to right the atomicradii will decrease• From the top to the bottom of a group, theatomic radii will increase• Draw arrows on your periodic table toindicate these trends
Atomic Radius• Atomic size is a periodic trend influencedby electron configuration.• For metals, atomic radius is half the distancebetween adjacent nuclei in a crystal of theelement.
Atomic Radius (cont.)• For elements that occur as molecules, theatomic radius is half the distance betweennuclei of identical atoms.
Atomic Radius (cont.)• There is a general decrease in atomicradius from left to right, caused byincreasing positive charge in the nucleus.• Valence electrons are not shielded from theincreasing nuclear charge because noadditional electrons come between thenucleus and the valence electrons.
Atomic Radius (cont.)
Atomic Radius (cont.)• Atomic radius generally increases as youmove down a group.• The outermost orbital size increases down agroup, making the atom larger.
Ionization Energy (cont.)• The octet rule states that atoms tend togain, lose or share electrons in order toacquire a full set of eight valence electrons.• The octet rule is useful for predicting whattypes of ions an element is likely to form.
Ionic Radius• An ion is an atom or bonded group ofatoms with a positive or negative charge.• When atoms lose electrons and formpositively charged ions, they always becomesmaller for two reasons:1. The loss of a valence electron can leave an emptyouter orbital resulting in a small radius.2. Electrostatic repulsion decreases allowing theelectrons to be pulled closer to the radius.
Ionic Radius (cont.)• When atoms gain electrons, they canbecome larger, because the addition of anelectron increases electrostatic repulsion.
Ionic Radius (cont.)• The ionic radii of positive ions generallydecrease from left to right.• The ionic radii of negative ions generallydecrease from left to right, beginning withgroup 15 or 16.
Ionic Radius (cont.)• Both positive and negative ions increase insize moving down a group.
Ionization Energy• Ion = an atom that has lost or gained anelectron causing it to have a positive ornegative charge• Ionization Energy = The energy required toremove the outermost electron from anatom• If an electron is pulled away from theatom, an ion is formed
Ionization Energy• Ionization energy is defined as the energyrequired to remove an electron from agaseous atom.• The energy required to remove the firstelectron is called the first ionization energy.
Ionization Energy (cont.)• Removing the second electron requiresmore energy, and is called the secondionization energy.• Each successive ionization requires moreenergy, but it is not a steady increase.
Ionization Energy (cont.)• The ionization at which the large increasein energy occurs is related to the number ofvalence electrons.• First ionization energy increases from left toright across a period.• First ionization energy decreases down agroup because atomic size increases andless energy is required to remove an electronfarther from the nucleus.
Ionization Energy (cont.)
Ionization Energy (cont.)
Ionization Energy (cont.)
Ionization Energy (cont.)
Metallic Character• Chemical properties associated withelements classified as metals• Examples??• As you move across the periodic tablefrom left to right, metallic characterdecreases• As you move down a group, the metalliccharacter increases
Metallic Character
Nonmetallic Character• Chemical propertiesassociated with chemicalsclassified as nonmetals• Examples???• As you move across theperiodic table from left toright, non metalliccharacter increases• As you move down agroup, non metalliccharacter decreases
Electronegativity• The measure of anatom’s ability to attractelectrons• As you move left to righton the periodic table,electronegativityincreases• As you move down agroup decreases due tothe longer distancebetween the outerelectrons and the nucleus
Ionization Energy (cont.)• The electronegativity of an elementindicates its relative ability to attractelectrons in a chemical bond.• Electronegativity decreases down a groupand increases left to right across a period.
Section 6.3 AssessmentThe lowest ionization energy is the ____.A. firstB. secondC. thirdD. fourthABA. AB. BC. C0% 0% 0% 0%D. DCD
Section 6.3 AssessmentThe ionic radius of a negative ionbecomes larger when:A. moving up a groupB. moving right to left across periodC. moving down a groupD. the ion loses electronsABA. AB. BC. C0% 0% 0% 0%D. DCD
Chemistry OnlineStudy Guide<strong>Chapter</strong> AssessmentStandardized Test PracticeImage BankConcepts in Motion
Key ConceptsSection 6.1 Development of theModern <strong>Periodic</strong> <strong>Table</strong>• The elements were first organized by increasingatomic mass, which led to inconsistencies. Later,they were organized by increasing atomic number.• The periodic law states that when the elements arearranged by increasing atomic number, there is aperiodic repetition of their chemical and physicalproperties.• The periodic table organizes the elements into periods(rows) and groups (columns); elements with similarproperties are in the same group.
Key ConceptsSection 6.1 Development of theModern <strong>Periodic</strong> <strong>Table</strong> (contd.)• Elements are classified as either metals, nonmetals,or metalloids.
Key ConceptsSection 6.2 Classification ofthe Elements• The periodic table has four blocks (s, p, d, f).• Elements within a group have similar chemicalproperties.• The group number for elements in groups 1 and 2equals the element’s number of valence electrons.• The energy level of an atom’s valence electrons equalsits period number.
Key ConceptsSection 6.3 <strong>Periodic</strong> Trends• Atomic and ionic radii decrease from left to rightacross a period, and increase as you move down agroup.• Ionization energies generally increase from left to rightacross a period, and decrease as you move down agroup.• The octet rule states that atoms gain, lose, or shareelectrons to acquire a full set of eight valence electrons.• Electronegativity generally increases from left to rightacross a period, and decreases as you move down agroup.
The actinide series is part of theA. s-block elements.B. inner transition metals.C. non-metals.D. alkali metals.ABA. AB. BC. C0% 0% 0% 0%D. DCD
In their elemental state, which group has acomplete octet of valence electrons?A. alkali metalsB. alkaline earth metalsC. halogensD. noble gasesABA. AB. BC. C0% 0% 0% 0%D. DCD
Which block contains the transitionmetals?A. s-blockB. p-blockC. d-blockD. f-blockABA. AB. BC. C0% 0% 0% 0%D. DCD
An element with a full octet has how manyvalence electrons?A. twoB. sixC. eightD. tenABA. AB. BC. C0% 0% 0% 0%D. DCD
How many groups of elements are there?A. 8B. 16C. 18D. 4ABA. AB. BC. C0% 0% 0% 0%D. DCD
Which group of elements are the leastreactive?A. alkali metalsB. inner transition metalsC. halogensD. noble gasesABA. AB. BC. C0% 0% 0% 0%D. DCD
On the modern periodic table, alkalineearth metals are found only in ____.A. group 1B. s-blockC. p-blockD. groups 13–18ABA. AB. BC. C0% 0% 0% 0%D. DCD
Unreactive gases are mostly found whereon the periodic table?A. halogensB. group 1 and 2C. group 18D. f-blockABA. AB. BC. C0% 0% 0% 0%D. DCD
Bromine is a member of theA. noble gases.B. inner transition metals.C. earth metals.D. halogens.ABA. AB. BC. C0% 0% 0% 0%D. DCD
How many groups does the d-block span?A. twoB. sixC. tenD. fourteenABA. AB. BC. C0% 0% 0% 0%D. DCD
Click on an image to enlarge.
<strong>Table</strong> 6.4Figure 6.5Noble Gas ElectronConfigurationThe <strong>Periodic</strong> <strong>Table</strong>Figure 6.11 Trends in Atomic RadiiFigure 6.18 Trends in Electronegativity
Click any of the background top tabsto display the respective folder.Within the <strong>Chapter</strong> Outline, clicking a sectiontab on the right side of the screen will bring youto the first slide in each respective section.Simple navigation buttons will allow you toprogress to the next slide or the previous slide.The <strong>Chapter</strong> Resources Menu will allow you toaccess chapter specific resources from the <strong>Chapter</strong>Menu or any <strong>Chapter</strong> Outline slide. From within anyfeature, click the Resources tab to return to this slide.The “Return” button will allow you to return to theslide that you were viewing when you clicked eitherthe Resources or Help tab.To exit the presentation, click the Exit button on the <strong>Chapter</strong> Menu slide or hitEscape [Esc] on your keyboards while viewing any <strong>Chapter</strong> Outline slide.
This slide is intentionally blank.