PRODUCT MONOGRAPH IMITREX DF IMITREX ... - GlaxoSmithKline
PRODUCT MONOGRAPH IMITREX DF IMITREX ... - GlaxoSmithKline PRODUCT MONOGRAPH IMITREX DF IMITREX ... - GlaxoSmithKline
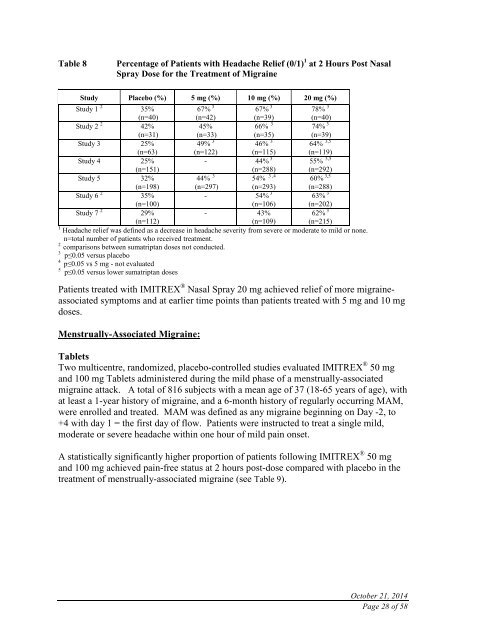
Table 8Percentage of Patients with Headache Relief (0/1) 1 at 2 Hours Post NasalSpray Dose for the Treatment of MigraineStudy Placebo (%) 5 mg (%) 10 mg (%) 20 mg (%)Study 1 2 35%(n=40)67% 3(n=42)67% 3(n=39)78% 3(n=40)Study 2 2 42%(n=31)45%(n=33)66% 3(n=35)74% 3(n=39)Study 3 25%(n=63)49% 3(n=122)46% 3(n=115)64% 3,5(n=119)Study 4 25%(n=151)- 44% 3(n=288)55% 3,5(n=292)Study 5 32%(n=198)44% 3(n=297)54% 3 ,4(n=293)60% 3,5(n=288)Study 6 2 35%(n=100)- 54% 3(n=106)63% 3(n=202)Study 7 2 29%(n=112)- 43%(n=109)62% 3(n=215)1 Headache relief was defined as a decrease in headache severity from severe or moderate to mild or none.n=total number of patients who received treatment.2comparisons between sumatriptan doses not conducted.3 p≤0.05 versus placebo4p≤0.05 vs 5 mg - not evaluated5 p≤0.05 versus lower sumatriptan dosesPatients treated with IMITREX ® Nasal Spray 20 mg achieved relief of more migraineassociatedsymptoms and at earlier time points than patients treated with 5 mg and 10 mgdoses.Menstrually-Associated Migraine:TabletsTwo multicentre, randomized, placebo-controlled studies evaluated IMITREX ® 50 mgand 100 mg Tablets administered during the mild phase of a menstrually-associatedmigraine attack. A total of 816 subjects with a mean age of 37 (18-65 years of age), withat least a 1-year history of migraine, and a 6-month history of regularly occurring MAM,were enrolled and treated. MAM was defined as any migraine beginning on Day -2, to+4 with day 1 = the first day of flow. Patients were instructed to treat a single mild,moderate or severe headache within one hour of mild pain onset.A statistically significantly higher proportion of patients following IMITREX ® 50 mgand 100 mg achieved pain-free status at 2 hours post-dose compared with placebo in thetreatment of menstrually-associated migraine (see Table 9).October 21, 2014Page 28 of 58
Table 9Percentage of Patients with Complete Headache Pain Relief 1 at 2 Hours PostOral Dose for the Treatment of Menstrually-Associated MigraineStudyPlacebo(%)50 mg(%)100 mg(%)Study 1 22(n=132)51*(n=138)58*(n=133)Study 2 29(n=118)51*(n=116)1 Complete Headache Pain Relief is defined as grade 1(mild pain) reduced to grade 0 (no pain).* p
- Page 3 and 4: IMITREX DF ®(sumatriptan succinate
- Page 5 and 6: Ergot-containing drugs have been re
- Page 7 and 8: The systematic approach described a
- Page 9 and 10: three of the subjects (two of whom
- Page 11 and 12: Special PopulationsPregnant Women:
- Page 13 and 14: e intense. These may occur in any p
- Page 15 and 16: Table 4Treatment-Emergent Adverse E
- Page 17 and 18: unaltered when preceded by a single
- Page 19 and 20: Clinical trials have shown that app
- Page 21 and 22: ACTION AND CLINICAL PHARMACOLOGYMec
- Page 23 and 24: STORAGE AND STABILITYIMITREX DF ®
- Page 25 and 26: Nasal SprayProper name:Chemical nam
- Page 27: Table 7Percentage of Patients with
- Page 31 and 32: Sumatriptan (1-1000 μg/kg, iv) pro
- Page 33 and 34: Metabolism of the methylaminosulpho
- Page 35 and 36: Chronic toxicity studies were carri
- Page 37 and 38: MutagenicitySumatriptan produced no
- Page 39 and 40: In inhalation toxicity studies (dog
- Page 41 and 42: 14. Heyck H. Pathogenesis of migrai
- Page 43 and 44: IMPORTANT: PLEASE READPART III: CON
- Page 45 and 46: IMPORTANT: PLEASE READOther side ef
- Page 47 and 48: IMPORTANT: PLEASE READPART III: CON
- Page 49 and 50: IMPORTANT: PLEASE READFigure 1• T
- Page 51 and 52: IMPORTANT: PLEASE READFigure 12How
- Page 53 and 54: IMPORTANT: PLEASE READSERIOUS SIDE
- Page 55 and 56: IMPORTANT: PLEASE READ• you are t
- Page 57 and 58: IMPORTANT: PLEASE READSIDE EFFECTS
Table 8Percentage of Patients with Headache Relief (0/1) 1 at 2 Hours Post NasalSpray Dose for the Treatment of MigraineStudy Placebo (%) 5 mg (%) 10 mg (%) 20 mg (%)Study 1 2 35%(n=40)67% 3(n=42)67% 3(n=39)78% 3(n=40)Study 2 2 42%(n=31)45%(n=33)66% 3(n=35)74% 3(n=39)Study 3 25%(n=63)49% 3(n=122)46% 3(n=115)64% 3,5(n=119)Study 4 25%(n=151)- 44% 3(n=288)55% 3,5(n=292)Study 5 32%(n=198)44% 3(n=297)54% 3 ,4(n=293)60% 3,5(n=288)Study 6 2 35%(n=100)- 54% 3(n=106)63% 3(n=202)Study 7 2 29%(n=112)- 43%(n=109)62% 3(n=215)1 Headache relief was defined as a decrease in headache severity from severe or moderate to mild or none.n=total number of patients who received treatment.2comparisons between sumatriptan doses not conducted.3 p≤0.05 versus placebo4p≤0.05 vs 5 mg - not evaluated5 p≤0.05 versus lower sumatriptan dosesPatients treated with <strong>IMITREX</strong> ® Nasal Spray 20 mg achieved relief of more migraineassociatedsymptoms and at earlier time points than patients treated with 5 mg and 10 mgdoses.Menstrually-Associated Migraine:TabletsTwo multicentre, randomized, placebo-controlled studies evaluated <strong>IMITREX</strong> ® 50 mgand 100 mg Tablets administered during the mild phase of a menstrually-associatedmigraine attack. A total of 816 subjects with a mean age of 37 (18-65 years of age), withat least a 1-year history of migraine, and a 6-month history of regularly occurring MAM,were enrolled and treated. MAM was defined as any migraine beginning on Day -2, to+4 with day 1 = the first day of flow. Patients were instructed to treat a single mild,moderate or severe headache within one hour of mild pain onset.A statistically significantly higher proportion of patients following <strong>IMITREX</strong> ® 50 mgand 100 mg achieved pain-free status at 2 hours post-dose compared with placebo in thetreatment of menstrually-associated migraine (see Table 9).October 21, 2014Page 28 of 58