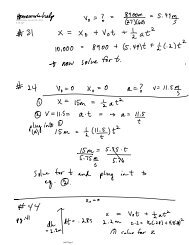
CSEC PHYSICAL SCIENCE LESSON 14 - Atomic Structure
CSEC PHYSICAL SCIENCE LESSON 14 - Atomic Structure
CSEC PHYSICAL SCIENCE LESSON 14 - Atomic Structure
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>CSEC</strong> <strong>PHYSICAL</strong> <strong>SCIENCE</strong><strong>LESSON</strong> <strong>14</strong> - <strong>Atomic</strong> <strong>Structure</strong> Know the different theories of the structure of the atom that have been proposed andwhich model is currently accepted.VOCABULARY / CONCEPTS- atomic model- Ancient Greek Model- Dalton’sTheory- Thomson Model- Rutherford’sTheory Ancient Greek ModelAristotle proposed an ‘Earth, Fire, Wind, Water’model to classify matter. Democritus thought there were ‘atoms’, or small particleswith unique properties that could not be divided.A r i s t o t l e
2 physci_lesson15_atomic_models.nb Dalton’s TheoryDalton proposed the theory that all matter is made up of individual particles called atoms, which cannot be divided. The mainpoints of Dalton’s theory are as follows:All elements are composed of atoms.All atoms of the same element have the same mass, and atoms of different elements have different masses.Compounds contain atoms of more than one element.In a particular compound, atoms of different elements always combine in the same way Thomson’s ModelThomson’s Model An atom is neutral, meaning it has neither a negative nor a positive charge. How can an atom contain negativeparticles and still be neutral? There must be some positive charge in the atom. In Thomson’s model of the atom, the negativecharges were evenly scattered throughout an atom filled with a positively charged mass of matter. The model is called the “plumpudding”model, after a traditional English dessert.
physci_lesson15_atomic_models.nb 3 Rutherford’s TheoryFrom http://library.thinkquest.org/19662/high/eng/exp-rutherford.html
4 physci_lesson15_atomic_models.nb Bohr ModelThe Bohr model, http://en.wikipedia.org/wiki/Bohr_model named after Niels Bohr, is a very important model for understandingenergy levels of atoms. It helped explain how certain energies were emitted (sometimes in the form of light, always in the form ofan electromagnetic wave).
physci_lesson15_atomic_models.nb 5 <strong>Atomic</strong> Orbitals Be able to distinguish between the three main subatomic particles that make up anatom.VOCABULARY / CONCEPTS- proton- electron- neutron
VOCABULARY / CONCEPTS- proton- electron- neutron6 physci_lesson15_atomic_models.nb Protons, Electrons, NeutronsThere are three main subatomic particles.Protons, Electrons, and Neutrons. The chart below shows the main distinctions between the particles, which are mass, charge, andlocation.Electrons can be added or lost from an atom at times. The number of protons determines which element it is. The number ofneutrons determines which isotope it is. The number of electrons determines if the atom will be an ion or remain neutral (whenthere are an equal number of protons and electrons).
physci_lesson15_atomic_models.nb 7 Know what quarks are, how they are observed, and what properties distinguish themapart.VOCABULARY / CONCEPTS- quark- up quark- down quark- particle acclerators QuarksA quark is an elementary particle and a fundamental constituent of matter. See wikipedia article here: http://en.wikipedia.org/wiki/QuarkThere are six main types of quarks. They vary by mass and average lifetime (when observed during particle collisions). One quark,the top quark, has a mass that is much larger than a proton. The two main types of quarks are up and down quarks. These areknown as first generation quarks.One proton is composed of two ‘up’quarks and one ‘down’quark.
8 physci_lesson15_atomic_models.nb
Particle Acceleratorphysci_lesson15_atomic_models.nb 9
10 physci_lesson15_atomic_models.nbParticle Accelerator