Revealing Early Steps of 2 1 Integrin-mediated Adhesion to ...
Revealing Early Steps of 2 1 Integrin-mediated Adhesion to ...
Revealing Early Steps of 2 1 Integrin-mediated Adhesion to ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Table 1. Percentage <strong>of</strong> rupture events (“j”) at single-molecule level (�73 pN) detected for different experimental conditions (inhibi<strong>to</strong>rs,<br />
contact times). The force interval (0,73 pN) encompasses 100% <strong>of</strong> the single-integrin unbinding events detected at comparable loading rates<br />
during single-molecule measurements<br />
Interval (pN)<br />
SMM<br />
(%)<br />
Short<br />
(%)<br />
Nonactivated,<br />
long (%)<br />
Activated,<br />
long (%)<br />
not be attributed <strong>to</strong> the rupture <strong>of</strong> single-integrin–collagen<br />
bonds.<br />
To compare the smallest rupture events between nonactivated<br />
and activated cells, we defined a force interval whose<br />
limits <strong>of</strong> 0 and 73 pN contained 100% <strong>of</strong> the single-integrin<br />
rupture forces measured during DFS. During the initiation<br />
phase (5–30 s), �90% <strong>of</strong> the small rupture events in nonactivated<br />
cells fell within this interval, and they were therefore<br />
consistent with the unbinding <strong>of</strong> single integrins from the<br />
collagen matrix (Table 1). During the reinforcement/maturation<br />
phase (120–300 s), the distribution <strong>of</strong> rupture forces<br />
was shifted slightly <strong>to</strong>ward higher forces. However, the<br />
majority <strong>of</strong> rupture events (75%) still lay within the singleintegrin<br />
unbinding force interval. We consequently concluded<br />
that in nonactivated cells, the majority <strong>of</strong> the smallest<br />
unbinding events represented the rupture <strong>of</strong> single-integrin–collagen<br />
bonds. In activated cells, only 26% <strong>of</strong> the<br />
small rupture events were within the single-integrin unbinding<br />
force interval. Thus, the increase in overall cell adhesion<br />
<strong>of</strong> activated cells coincided with the frequent rise <strong>of</strong> the<br />
smallest force units above the single-integrin level. In these<br />
cells, the smallest force units showed a wide distribution<br />
and reached values as high as 700 pN, or �15 times the<br />
value determined for the unbinding <strong>of</strong> a single integrin. The<br />
rupture force increase over the single-integrin level suggested<br />
that functional adhesive units containing varying<br />
numbers <strong>of</strong> � 2� 1 recep<strong>to</strong>rs had formed and that these recep<strong>to</strong>rs<br />
displayed cooperative binding.<br />
Ac<strong>to</strong>myosin Contractility Is Required for Establishing<br />
<strong>Integrin</strong> Cooperativity and Overall Cell <strong>Adhesion</strong><br />
In cells establishing substrate contact, integrins are frequently<br />
clustered in<strong>to</strong> small, dot-like focal complexes that<br />
may later mature in<strong>to</strong> larger focal adhesion structures. The<br />
maturation <strong>of</strong> early adhesive sites in<strong>to</strong> focal adhesions involves<br />
myosin II-driven contractility regulated by the small<br />
GTPase RhoA and its effec<strong>to</strong>rs ROCK (Rottner et al., 1999).<br />
RhoA activity is also required <strong>to</strong> bundle ac<strong>to</strong>myosin filaments<br />
in<strong>to</strong> large stress fibers that terminate in focal adhesion<br />
sites (Chrzanowska-Wodnicka and Burridge, 1996). Therefore,<br />
there is a clear link between ac<strong>to</strong>myosin-driven contractility,<br />
the formation <strong>of</strong> mature integrin adhesion complexes,<br />
and the establishment <strong>of</strong> strong overall cell adhesion.<br />
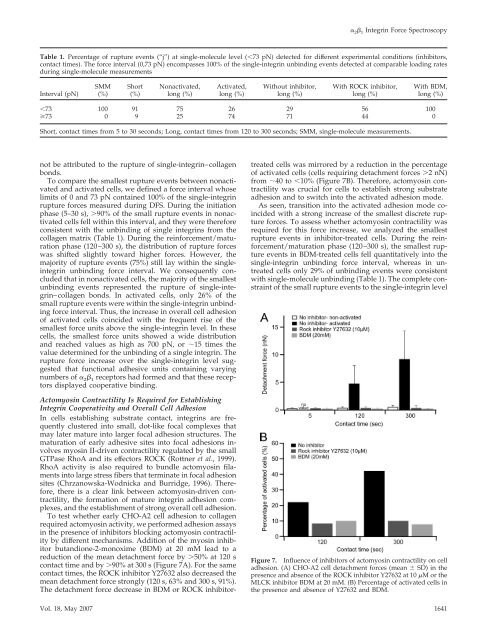
To test whether early CHO-A2 cell adhesion <strong>to</strong> collagen<br />
required ac<strong>to</strong>myosin activity, we performed adhesion assays<br />
in the presence <strong>of</strong> inhibi<strong>to</strong>rs blocking ac<strong>to</strong>myosin contractility<br />
by different mechanisms. Addition <strong>of</strong> the myosin inhibi<strong>to</strong>r<br />
butandione-2-monoxime (BDM) at 20 mM lead <strong>to</strong> a<br />
reduction <strong>of</strong> the mean detachment force by �50% at 120 s<br />
contact time and by �90% at 300 s (Figure 7A). For the same<br />
contact times, the ROCK inhibi<strong>to</strong>r Y27632 also decreased the<br />
mean detachment force strongly (120 s, 63% and 300 s, 91%).<br />
The detachment force decrease in BDM or ROCK inhibi<strong>to</strong>r-<br />
Without inhibi<strong>to</strong>r,<br />
long (%)<br />
� 2� 1 <strong>Integrin</strong> Force Spectroscopy<br />
With ROCK inhibi<strong>to</strong>r,<br />
long (%)<br />
With BDM,<br />
long (%)<br />
�73 100 91 75 26 29 56 100<br />
�73 0 9 25 74 71 44 0<br />
Short, contact times from 5 <strong>to</strong> 30 seconds; Long, contact times from 120 <strong>to</strong> 300 seconds; SMM, single-molecule measurements.<br />
treated cells was mirrored by a reduction in the percentage<br />
<strong>of</strong> activated cells (cells requiring detachment forces �2 nN)<br />
from �40 <strong>to</strong> �10% (Figure 7B). Therefore, ac<strong>to</strong>myosin contractility<br />
was crucial for cells <strong>to</strong> establish strong substrate<br />
adhesion and <strong>to</strong> switch in<strong>to</strong> the activated adhesion mode.<br />
As seen, transition in<strong>to</strong> the activated adhesion mode coincided<br />
with a strong increase <strong>of</strong> the smallest discrete rupture<br />
forces. To assess whether ac<strong>to</strong>myosin contractility was<br />
required for this force increase, we analyzed the smallest<br />
rupture events in inhibi<strong>to</strong>r-treated cells. During the reinforcement/maturation<br />
phase (120–300 s), the smallest rupture<br />
events in BDM-treated cells fell quantitatively in<strong>to</strong> the<br />
single-integrin unbinding force interval, whereas in untreated<br />
cells only 29% <strong>of</strong> unbinding events were consistent<br />
with single-molecule unbinding (Table 1). The complete constraint<br />
<strong>of</strong> the small rupture events <strong>to</strong> the single-integrin level<br />
Figure 7. Influence <strong>of</strong> inhibi<strong>to</strong>rs <strong>of</strong> ac<strong>to</strong>myosin contractility on cell<br />
adhesion. (A) CHO-A2 cell detachment forces (mean � SD) in the<br />
presence and absence <strong>of</strong> the ROCK inhibi<strong>to</strong>r Y27632 at 10 �M orthe<br />
MLCK inhibi<strong>to</strong>r BDM at 20 mM. (B) Percentage <strong>of</strong> activated cells in<br />
the presence and absence <strong>of</strong> Y27632 and BDM.<br />
Vol. 18, May 2007 1641