Large Volume Inorganic Chemicals - Ammonia ... - ammk-rks.net
Large Volume Inorganic Chemicals - Ammonia ... - ammk-rks.net Large Volume Inorganic Chemicals - Ammonia ... - ammk-rks.net
Chapter 33.2.7 Production of concentrated nitric acidDirect processes for the production of concentrated nitric acid are based on the production ofliquid N 2 O 4 , which reacts under pressure with oxygen and dilute nitric acid to form HNO 3 .2 N 2 O 4 + O 2 + 2 H 2 O f 4 HNO 3Nitrogen oxides, which are formed in an ammonia combustion unit operated at low pressure, arecompletely oxidised into NO 2 (oxidation step and post-oxidation step). NO 2 is washed out withconcentrated nitric acid (absorption step) and by process condensate and dilute nitric acid (finalabsorber step). NO 2 (or its dimer N 2 O 4 ) is stripped from the concentrated acid (bleaching) andliquefied. Concentrated acid is formed from liquid N 2 O 4 , oxygen and dilute nitric acid (from thefinal absorption step) in a reactor at a pressure of approximately 50 bar. Concentrated nitric acidis recirculated to the absorption and final oxidation steps, part of nitric acid is withdrawn asproduct acid. Waste gas is discharged from the final absorption step. NO x concentration of thewaste gas depends on the temperature of the final absorption step. Process condensates anddilute nitric acid arising from catalytic ammonia oxidation, oxidation and post oxidation step arere-used. However, more process condensate is formed than can be used for production ofconcentrated acid. A part of the process condensates has to be used for other production ortreated as waste water.Indirect processes are based on extractive distillation and rectification of weak nitric acid.Sulphuric acid or magnesium nitrate is used as the dehydrating agent. With sulphuric acidprocesses, weak nitric acid is preheated and distilled with H 2 SO 4 . With magnesium nitrateprocesses, a solution of Mg(NO 3 ) 2 is used to extract water from the nitric acid. Dehydratingagents are restored under vacuum. Process condensates which demand an adequate waste watertreatment arise from concentrating the dehydrating agent. Vapour withdrawn from the head ofthe distillation or extraction column is condensed to form concentrated nitric acid. Waste gasescontain nitric acid vapour and are scrubbed with dilute nitric acid.100 December 2006 BS/EIPPCB/LVIC-AAF_BREF_FINAL
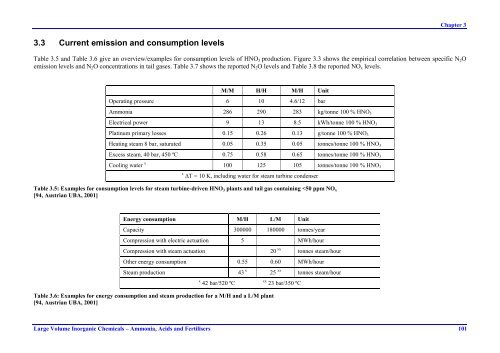
Chapter 33.3 Current emission and consumption levelsTable 3.5 and Table 3.6 give an overview/examples for consumption levels of HNO 3 production. Figure 3.3 shows the empirical correlation between specific N 2 Oemission levels and N 2 O concentrations in tail gases. Table 3.7 shows the reported N 2 O levels and Table 3.8 the reported NO x levels.M/M H/H M/H UnitOperating pressure 6 10 4.6/12 barAmmonia 286 290 283 kg/tonne 100 % HNO 3Electrical power 9 13 8.5 kWh/tonne 100 % HNO 3Platinum primary losses 0.15 0.26 0.13 g/tonne 100 % HNO 3Heating steam 8 bar, saturated 0.05 0.35 0.05 tonnes/tonne 100 % HNO 3Excess steam, 40 bar, 450 ºC 0.75 0.58 0.65 tonnes/tonne 100 % HNO 3Cooling water x 100 125 105 tonnes/tonne 100 % HNO 3x jT = 10 K, including water for steam turbine condenserTable 3.5: Examples for consumption levels for steam turbine-driven HNO 3 plants and tail gas containing
- Page 78 and 79: Chapter 2Production process Feedsto
- Page 80 and 81: Chapter 22.3.2 NO x emissionsTable
- Page 82 and 83: Chapter 22.3.3 Other consumption le
- Page 84 and 85: Chapter 2ParameterProcessEmission l
- Page 86 and 87: Chapter 22.4 Techniques to consider
- Page 88 and 89: Chapter 22.4.2 Processes with reduc
- Page 90 and 91: Chapter 22.4.3 Heat exchange autoth
- Page 92 and 93: Chapter 22.4.4 Revamp: increase cap
- Page 94 and 95: Chapter 22.4.5 Pre-reformingDescrip
- Page 96 and 97: Chapter 2ApplicabilityGenerally app
- Page 98 and 99: Chapter 22.4.7 Advanced process con
- Page 100 and 101: Chapter 22.4.9 Combined Claus unit
- Page 102 and 103: Chapter 2Operational dataSee Descri
- Page 104 and 105: Chapter 22.4.12 Preheating of combu
- Page 106 and 107: Chapter 22.4.14 Isothermal shift co
- Page 108 and 109: Chapter 22.4.16 Stripping and recyc
- Page 110 and 111: Chapter 22.4.18 Use of sulphur resi
- Page 112 and 113: Chapter 22.4.20 Indirect cooling of
- Page 114 and 115: Chapter 22.4.22 Ammonia removal fro
- Page 116 and 117: Chapter 22.4.24 Metal recovery and
- Page 118 and 119: Chapter 2Driving force for implemen
- Page 120 and 121: Chapter 22.5 BAT for ammoniaBAT is
- Page 123 and 124: Chapter 33 NITRIC ACID3.1 General i
- Page 125 and 126: Chapter 3Pressure in bar Temperatur
- Page 127: Chapter 33.2.5 Tail gas properties
- Page 131 and 132: Chapter 3N 2 O emission levelProces
- Page 133 and 134: Chapter 3N 2 O emission levelProces
- Page 135 and 136: Chapter 3Process typeNO x emission
- Page 137 and 138: Chapter 3Process typeNO x emission
- Page 139 and 140: Chapter 3140120Generation factor %1
- Page 141 and 142: Chapter 33.4.2 Optimisation of the
- Page 143 and 144: Chapter 33.4.3 Alternative oxidatio
- Page 145 and 146: Chapter 33.4.4 Optimisation of the
- Page 147 and 148: Chapter 3Achieved environmental ben
- Page 149 and 150: Chapter 33.4.5 N 2 O decomposition
- Page 151 and 152: Chapter 33.4.6 Catalytic N 2 O deco
- Page 153 and 154: Chapter 3According to [89, Kuiper,
- Page 155 and 156: Chapter 33.4.7 Combined NO x and N
- Page 157 and 158: Chapter 3EconomicsInvestment costs.
- Page 159 and 160: Chapter 3Operational dataSee descri
- Page 161 and 162: Chapter 3NOx removal efficiency in
- Page 163 and 164: Chapter 33.4.10 Addition of H 2 O 2
- Page 165 and 166: Chapter 33.4.11 NO X reduction duri
- Page 167 and 168: Chapter 3Installing a low temperatu
- Page 169 and 170: Chapter 3NO x emission level as NO
- Page 171: Chapter 3Operational dataNo specifi
- Page 174 and 175: Chapter 4Country Company Location C
- Page 176 and 177: Chapter 4Country Company Location C
Chapter 33.3 Current emission and consumption levelsTable 3.5 and Table 3.6 give an overview/examples for consumption levels of HNO 3 production. Figure 3.3 shows the empirical correlation between specific N 2 Oemission levels and N 2 O concentrations in tail gases. Table 3.7 shows the reported N 2 O levels and Table 3.8 the reported NO x levels.M/M H/H M/H UnitOperating pressure 6 10 4.6/12 bar<strong>Ammonia</strong> 286 290 283 kg/tonne 100 % HNO 3Electrical power 9 13 8.5 kWh/tonne 100 % HNO 3Platinum primary losses 0.15 0.26 0.13 g/tonne 100 % HNO 3Heating steam 8 bar, saturated 0.05 0.35 0.05 tonnes/tonne 100 % HNO 3Excess steam, 40 bar, 450 ºC 0.75 0.58 0.65 tonnes/tonne 100 % HNO 3Cooling water x 100 125 105 tonnes/tonne 100 % HNO 3x jT = 10 K, including water for steam turbine condenserTable 3.5: Examples for consumption levels for steam turbine-driven HNO 3 plants and tail gas containing