Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
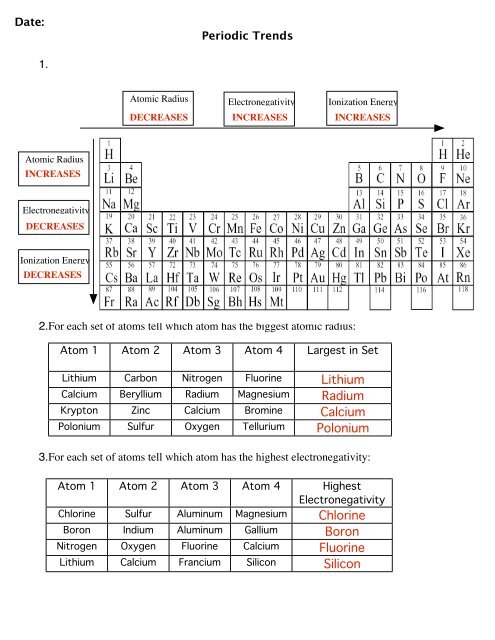
Date:Periodic <strong>Trends</strong>1.Atomic Radius Electronegativity Ionization EnergyDECREASESINCREASESINCREASESAtomic RadiusINCREASESElectronegativityDECREASESIonization EnergyDECREASES2.For each set of atoms tell which atom has the biggest atomic radius:Atom 1 Atom 2 Atom 3 Atom 4 Largest in SetLithium Carbon Nitrogen Fluorine LithiumCalcium Beryllium Radium Magnesium RadiumKrypton Zinc Calcium Bromine CalciumPolonium Sulfur Oxygen Tellurium Polonium3.For each set of atoms tell which atom has the highest electronegativity:Atom 1 Atom 2 Atom 3 Atom 4 HighestElectronegativityChlorine Sulfur Aluminum Magnesium ChlorineBoron Indium Aluminum Gallium BoronNitrogen Oxygen Fluorine Calcium FluorineLithium Calcium Francium Silicon Silicon
Date:Periodic <strong>Trends</strong>4.For each set of atoms tell which atom has the lowest ionization energy.Atom 1 Atom 2 Atom 3 Atom 4 Lowest IonizationEnergyNitrogen Fluorine Oxygen Carbon CarbonIodine Bromine Fluorine Chlorine IodineLithium Beryllium Boron Carbon LithiumAluminum Silicon Phosphorus Sulfur Aluminum5.Which would be larger the ionic radius or the atomic radius?a. Calcium’s Atomic Radius or Ionic RadiusCalcium’s ionic radius is smaller because it losses two electrons and an energy level.Therefore, the atomic radius is larger.b. Fluorine’s Atomic Radius or Ionic RadiusFluorine’s ionic radius is larger because it gains one electrons. This means it now hasmore electrons than protons making it harder to hold in the electrons and making the sizeslightly larger.c. Bromine’s Atomic Radius or Ionic RadiusBromine’s ionic radius is larger because it gains one electrons. This means it now hasmore electrons than protons making it harder to hold in the electrons and making the sizeslightly larger.d. Potassium’s Atomic Radius or Ionic RadiusPotassium’s ionic radius is smaller because it losses one electrons and an energy level.Therefore, the atomic radius is larger.