Electronic absorption spectra Allowed vs forbidden transitions
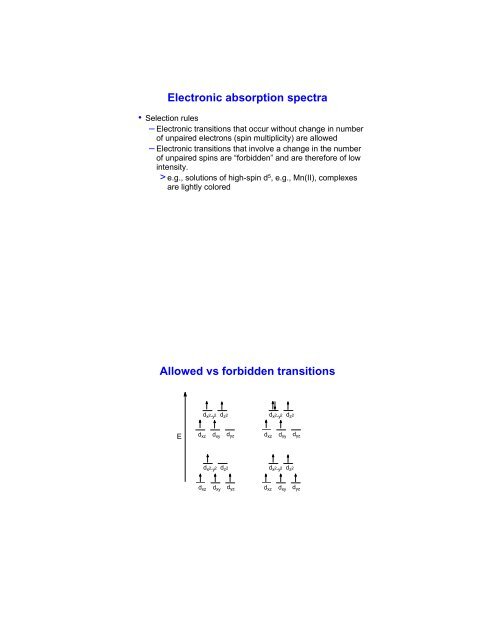
Electronic absorption spectra Allowed vs forbidden transitions
Electronic absorption spectra Allowed vs forbidden transitions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Transitions in d 1 and d 2 complexesEd xz d xy d yzd x 2 -y 2 d z 2 d xz d xy d yzd x 2 -y 2 d z 2 d x 2 -y 21d xzd xyd yzd x 2 -y 2 d z 2 d x 2 -y 2d z 2d xz d xy d yz1d xy d x 2 -y 21d xz1d xz 1 d z 2 1 d yz 1 d z 2 1d xy 1 d z 2 1 d yz 1 d x 2 -y 21d xz d xy d yzd x 2 -y 2 d z 2d x 2 -y 2 d z 2d xz d xy d yzSome compounds are very highly coloredbecause of charge transfer <strong>transitions</strong> – evensome with no d electrons● There can be electronic <strong>transitions</strong> in the visible region that donot involved d-electrons− MnO 4- (purple) and CrO 42-(yellow) are intensely coloredbecause electrons in filled oxygen based orbitals areexcited into empty d-orbitals (LMCT)− Ligand to Metal Charge Transfer (LMCT) bands have fewselection rule restrictions and are typically very intense● Metal to Ligand Charge Transfer (MLCT) bands may also occurfor complexes with d-electrons.− There are few selection rules and the high intensity of thesebands may mask d-d <strong>transitions</strong>.
Crystal field splitting in tetrahedral complexes• Tetrahedral arrangement of four ligandsshowing their orientation relative to theCartesian axes and the d yzorbital.• The orientation with respect to d xz, d xzandd xyis identical and the interaction withthese orbitals is considerably greater thanwith the d z2and d x2-y2orbitals; thereforethe d yz, d xzand d xyorbitals are higher inenergy than d z2and d x2-y2.• Because there are only four ligands andthe ligand electron pairs do not pointdirectly at the orbitals, Δ t~4/9 Δ o. As aresult the spin-pairing energy is alwaysgreater than Δ and tetrahedral complexesare always high spin.Comparison of crystal field splittings foroctahedral, square planar and tetrahedralligand fields
Factors affecting the magnitude of ) (CrystalField Splitting)● Charge on the metal. For first row transition elements ) Ovaries fromabout 7,500 cm -1 to 12,500 cm -1 for divalent ions and 14,000 cm -1 to25,000 cm -1 for trivalent ions.● Position in a group. ) Ovalues for analogous complexes of metal ionsin a group increase by 25% to 50% on going from one transition seriesto the next. This is illustrated by the complexes [M(NH 3) 6] 3+ where )values are 23,000 cm -1 for M=Co; 34,000 cm -1 for M=Rh and 41,000cm -1 for M=Ir.● Geometry and coordination number. For similar ligands ) twill beabout 4/9 ) O. This is a result of the reduced number of ligands andtheir orientation relative to the d orbitals. Recall that the energyordering of the orbitals is reversed in tetrahedral complexes relative tothat in the octahedral case.● Identity of the ligand. The dependence of ) on the nature of theligand follows a regular order, known as the spectrochemical series, forall metals in all oxidation states and geometries.