entire issue [pdf 12.7 mb] - Pitt Med - University of Pittsburgh
entire issue [pdf 12.7 mb] - Pitt Med - University of Pittsburgh
entire issue [pdf 12.7 mb] - Pitt Med - University of Pittsburgh
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
UNIVERSITY OF PITTSBURGH SCHOOL OF MEDICINE | FALL 2012PITTMEDMISSING LINKSPITT DOCS CAN NOW SEE WHEREBRAIN NETWORKS ARE CUT OFF
OVER THE TRANSOMA FAIR GRADECongratulations to my grandniece, Jenelle Pifer,for her wonderful reme<strong>mb</strong>rance <strong>of</strong> Dr. Hooker[“The Great Equalizer,” Summer 2012]. Inthose days we had tests known as “rat races” inwhich we were to identify tagged parts <strong>of</strong> thehuman body laid out on a table. I failed one. Dr.Hooker later called me into his <strong>of</strong>fice and said,“I realized you were sick that day, and I shouldhave told you to go home and rest. Your gradesin anatomy have always been high, and I’m notgoing to allow one bad grade to destroy yourrecord. Therefore, I’m throwing it out!” I wasvery touched by his awareness and caring.Charles Pifer (MD ’56)Carmel, Calif.RECENT MAGAZINEHONORS2012 AAMC Robert G. FenleyWriting Award for Excellence,Basic Science, Staff Writing(E. Vitone, “Mars and VenusRevisited”)2011 CASE, District IIGold, Covers (“None <strong>of</strong> My MemoriesAre My Own,” design by E. Cerri)2011 CASE, District IISilver, Staff Writing2011 IABC Golden Triangle Award<strong>of</strong> Excellence, Feature Writing(E. Vitone, “Mars and VenusRevisited”)2011 IABC Golden TriangleAward <strong>of</strong> Honor, MagazinesWe gladly receive letters (which we mayedit for length, style, and clarity).<strong>Pitt</strong> <strong>Med</strong>400 Craig Hall<strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh<strong>Pitt</strong>sburgh, PA 15260Phone: 412-624-4358Fax: 412-624-1021E-mail: medmag@pitt.eduhttp://pittmed.health.pitt.eduSTEP RIGHT UPOkay, maybe your life feels likea three-ring circus. But you canspare a minute to catch us up onyour doings, right? Tell us aboutyour career advancements, honorsyou’ve received, appointments,volunteer work, publications, andother death-defying acts. And welove to hear old <strong>Pitt</strong> memories.For instance, what’s the story withthis uni-doc we found in <strong>Pitt</strong>’s1970 Hippocratean? Quit clowningaround and drop us a line at thecontact info listed to the right, orfriend us on Facebook at www.pittmedfb.pitt.edu/.
PITTMEDUNIVERSITY OF PITTSBURGH SCHOOL OF MEDICINE MAGAZINE, FALL 2012 (SEPT/OCT/NOV)VOL. 14, ISSUE 36OF NOTE 3A home for personalized medicine.New program trains docs infamily medicine and psychiatry.CLOSER 7<strong>Pitt</strong> students are good newsfor local broadcaster.32INVESTIGATIONS 8Lymphedema unveiled.The genetic “captain” <strong>of</strong> fibroids.The underpinnings <strong>of</strong> gestational diabetes.ATTENDING 32Summer stories.98.6 DEGREES 35Lemieux comes throughfor blood cancers.ALUMNI NEWS 36A wealth <strong>of</strong> Dunmires.Charles Cochrane’s newdrug will save newborns.LAST CALL 40That’s not natural.FEATURESEvery Movement Counts 12Neurosurgeon and basic scientist Robert Friedlander, who makes astonishinglygood use <strong>of</strong> his time, says <strong>Pitt</strong> has everything he was looking for in achair opportunity.BY DAVID R. ELTZ27CONTRIBUTORSSUDHIR PATHAK and KEVIN JARBO [cover, “Missing Links”] are two <strong>of</strong> the figures behindwhy the new enhanced MRI technique called high-definition fiber tracking (HDFT) works, and whyit looks so cool. Pathak studied mathematics and scientific computing in India and later receiveda master’s in biomedical engineering from Carnegie Mellon <strong>University</strong>. As a programmer in theSchneider Lab at <strong>Pitt</strong>, he developed HDFT’s complex algorithms and now enjoys exploring howinformation and art intersect. HDFT is “art constrained by information,” he says. Pathak finds thescans so visually compelling he’s been using his free time to adapt them into original artwork;his pieces are featured in the online gallery <strong>of</strong> the Organization for Human Brain Mapping. Jarbois more interested in the scans’ clinical applications. He received his bachelor’s in biology at <strong>Pitt</strong>in 2006. Now, as assistant researcher in the lab, he helps doctors read the scans properly. HDFTfinally does the organ justice. “The reason why it ends up looking so cool,” he says, “is becausethe brain actually looks that cool.”COVERRThe brain, in all its connective glory, revealed. (Cover: Schneider Lab © 2012.)40Missing Links 16New technology, developed at <strong>Pitt</strong>, tracks the physical connectionsthat allow the brain to work. It’s already helping patients and neurosurgeons.COVER STORY BY JENELLE PIFERMitochondria’s Many Missions 22Addicts, Parkinson’s, ischemia/reperfusion injury, and very sick flies.Mitochondria tie them together.BY JOE MIKSCHSurvival <strong>of</strong> the Funded 27Nationally, the ecosystems that support getting scientific discoveries into theclinic have been pretty fragile. A few years ago, the NIH stepped in to help.<strong>Pitt</strong>’s Clinical and Translational Science Institute has helped create a healthyenvironment here.BY ELAINE VITONE
DEAN’S MESSAGEPITTMEDPUBLISHERArthur S. Levine, MDEDITOR IN CHIEFErica LloydART DIRECTORElena Gialamas CerriSENIOR EDITORJoe MikschASSOCIATE EDITORElaine VitoneGUEST EDITORJenelle PiferCONTRIBUTING EDITORChuck StaresinicPRODUCTION MANAGERChuck DinsmoreSTAFF CONTRIBUTORSEm Maier, Hayavadhan ThuppalOBITUARIES CONTRIBUTORMacy Levine, MD ’43CIRCULATION MANAGERCrystal KubicEDITORIAL ADVISORSJeremy Berg, PhDMichelle Broido, PhDNancy Davidson, MDPaula Davis, MASusan Dunmire, MD ’85, Res ’88Joan Harvey, MDSteven Kanter, MDDavid Kupfer, MDJoan Lakoski, PhDDavid Lewis, MDMargaret C. McDonald, PhD, MFADavid Perlmutter, MDSteven Shapiro, MDPeter Strick, PhDBennett Van Houten, PhDSimon Watkins, PhDMarshall Webster, MD, Res ’70Julius Youngner, ScDOFFICE OF PUBLIC AFFAIRSRobert HillJohn D. Harvith, JD<strong>Pitt</strong> <strong>Med</strong> is published by the Office <strong>of</strong> the Dean and Senior ViceChancellor for the Health Sciences in cooperation with the publicaffairs <strong>of</strong>fice. It is produced quarterly for alumni, students, staff,faculty, and friends <strong>of</strong> the School <strong>of</strong> <strong>Med</strong>icine. PR6721The <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh is an affirmative action, equalopportunity institution. © 2012, <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh<strong>Pitt</strong> med alumnus Gordon Sun(MD ’06), a Robert Wood JohnsonClinical Scholar at the <strong>University</strong><strong>of</strong> Michigan, details in an August New EnglandJournal <strong>of</strong> <strong>Med</strong>icine paper how emerging Asianpowers are ramping up national funding for medicalresearch and development; they are poised t<strong>of</strong>ill the void being created by contracting federalU.S. funding. The NIH’s proposed FY 2013budget continues the 10-year failure to matchthe increasing cost and opportunities <strong>of</strong> medicalresearch. Unless Congress changes its course, the debt reduction “sequestration” would leadto the closure <strong>of</strong> many good labs and the end <strong>of</strong> many careers, especially those <strong>of</strong> younginvestigators.Of course, China, India, South Korea, et al. have some catching up to do. The UnitedStates has held a premier position in medical research for more than half a century; it certainlywon’t be outpaced overnight. But perhaps the words “catching up” and “outpace”evoke the wrong images. Science is not a horse race—it’s a global collaboration. As othersinvest in acquiring knowledge, we all benefit. Yet I admit that I’d prefer that our countryalso continue to value medical research as an engine for innovative care and economicgrowth. Our federal investment in medical research is less than 1 percent <strong>of</strong> our GDP; theAsian powers invest 2 to 5 percent, with as much as a 67 percent increase, year to year.Already this is leading to the transfer <strong>of</strong> talent and resources from our shores. Our nation islikely to suffer a loss <strong>of</strong> jobs, access to experimental treatments, and other, more difficult tomeasure, windfalls that accompany a commitment to inquiry and innovation.Still, this is an exciting time here at home. Our medical school’s extraordinary rise inprominence continues. Yet another stellar class has joined us this August; we also welcomedsome <strong>of</strong> China’s best and brightest to our campus—and we are pleased to have taken thisstep in global collaboration. These young people will be working with our faculty as thefirst group to pursue a new, required, two-year biomedical research component <strong>of</strong> themedical school curriculum <strong>of</strong> Tsinghua <strong>University</strong> (“the MIT <strong>of</strong> China”). Yigong Shi, deanat Tsinghua (and a renowned structural biologist lured back to China from Princeton), haspartnered with us so that Tsinghua students will be immersed in Western research cultureand the critical thinking it promotes—as he was during his own U.S. graduate training.While Chinese students <strong>of</strong>ten gain a deep and impressive understanding <strong>of</strong> their subjects,their academic tradition does not encourage them to challenge scientific dogma.When considering such cultural and philosophic nuances, I was introduced to this passagepenned by an 8th-century Taoist poet: Wild geese fl y across the long sky above./Theirimage is refl ected upon the chilly water below./The geese do not mean to cast their image onthe water;/Nor does the water mean to hold the image <strong>of</strong> the geese. Although I confess to alimited knowledge <strong>of</strong> classical Chinese poetic form, I think that the reflection <strong>of</strong> geese canbe understood as a metaphor for the <strong>of</strong>ten serendipitous nature <strong>of</strong> scientific observation andthe creativity inherent in how we capture the accidental observation.I look forward to the serendipity and creativity ahead. And to watching our studentssoar—whether they started their journeys by flying across the long sky from Beijing or busingit on the 61B from Braddock.Arthur S. Levine, MDSenior Vice Chancellor for the Health SciencesDean, School <strong>of</strong> <strong>Med</strong>icineJOSHUA FRANZOS2 PITTMED
OF NOTEDevoted to noteworthy happeningsat the medical schoolTAKING MEDICINE PERSONALLYThe nu<strong>mb</strong>ers are big: $300 million, 350,000 square feet,375 jobs. So is the focus: to delve into the inheritedand environmental factors that account for individualsusceptibility to illness. Planning is under way for theUPMC Center for Innovative Science. Officials are evaluatingwhat was the Ford Motor Co. building—which sitson Baum Boulevard near the Hillman Cancer Center andUPMC Shadyside—as a potential site. The new center willgive <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh School <strong>of</strong> <strong>Med</strong>icine facultyme<strong>mb</strong>ers a base for work they hope will lead to tailormadetreatments. —Joe MikschZGF ARCHITECTSA rendering <strong>of</strong> the UPMC Center for Innovative Science, at a proposed site in Shadyside.FOOTNOTEWorld Wrestling Entertainment star TheGreat Khali expects to return to the ring followinga successful operation this summer at UPMC Presbyterianto remove a tumor from his pituitary gland. The tumorcaused surplus production <strong>of</strong> growth hormone, leading to acondition known as acromegaly, characterized by excessivegrowth in muscles and organs. The Great Khali, whose realname is Dalip Singh, finds such symptoms <strong>of</strong> use in hiswrestling career—as did the late André the Giant. KhaliCritical SuccessDespite being only a decade old, the <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh’s Department<strong>of</strong> Critical Care <strong>Med</strong>icine—believed to be the nation’s first—recentlyreceived a significant acknowledgment <strong>of</strong> its influence. Of 20 intensivecarespecialists granted the new title <strong>of</strong> Master in Critical Care <strong>Med</strong>icineFellow, the Society <strong>of</strong> Critical Care <strong>Med</strong>icine’s highest honorific, sevenwere former or current faculty <strong>of</strong> <strong>Pitt</strong>’s ahead-<strong>of</strong>-its-time department.Three <strong>of</strong> the new fellows represent the legacy <strong>of</strong> intensive-careresearch at <strong>Pitt</strong>: Mitchell Fink, an MD and founding chair <strong>of</strong> the department;emeritus pr<strong>of</strong>essor Ake Grenvik, an MD/PhD; and legendary intensive-carephysician and researcher Peter Safar, who died in 2003 and wasbestowed the title posthumously.Four current faculty me<strong>mb</strong>ers also were named fellows: departmentchair and pr<strong>of</strong>essor Derek Angus, who is an MD/MPH, and MDs PatrickKochanek (pr<strong>of</strong>essor and vice chair, as well as director <strong>of</strong> <strong>Pitt</strong>’s SafarCenter for Resuscitation Research) and pr<strong>of</strong>essors Michael Pinsky andAnn Thompson. —Justin Hopperstands 7-foot-1 and weighs 347 pounds.FALL 2012 3
Faculty SnapshotsEdward ChuOn Herbal Relief from ChemotherapyIn an open-air market in China, locals might be stocking their kitchens or grabbing a snack; theymight also be visiting a doctor, <strong>of</strong>ten a practitioner <strong>of</strong> both Western and traditional Chinese medicine.After an on-the-spot exam, if the complaint is <strong>of</strong> belly aches, nausea, or diarrhea, the physicianmight prescribe an herbal formulation called Huang Qin Tang, serving it as a tea or a smoothie.<strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh pr<strong>of</strong>essor Edward Chu (shown above), an MD who is deputy director <strong>of</strong>the <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh Cancer Institute, chief <strong>of</strong> <strong>Pitt</strong>’s Division <strong>of</strong> Hematology/Oncology, andan associate director <strong>of</strong> <strong>Pitt</strong>’s Drug Discovery Institute, and his colleague, Yung-chi (Tommy) Cheng,a PhD pharmacologist at Yale <strong>University</strong>, have tested the herbal compound in animal and blind clinicalpilot studies. The preliminary studies showed it <strong>of</strong>fers pr<strong>of</strong>ound relief from gastrointestinal ailmentsfor patients undergoing various chemotherapies. Huang Qin Tang has been used to treat suchailments for nearly 2,000 years.It may even help fight certain cancers. Chu and Cheng’s work is supported by the NationalCancer Institute’s Office <strong>of</strong> Cancer Complementary and Alternative <strong>Med</strong>icine.Why they took on the studiesWe’ve seen friends, relatives, and colleagues who’ve had a variety <strong>of</strong> cancers experience significantside effects <strong>of</strong> traditional Western medicines; and unfortunately, their disease continues to progress.Then they’ll be treated with these herbal Chinese medicines and have some pretty dramaticresponses—either alone or when used with chemotherapy. Their quality <strong>of</strong> life seemed to be better,and their toxicity was diminished. And since both <strong>of</strong> us are <strong>of</strong> Chinese origin, we said, “Maybe weneed to study this in a scientifically rigorous fashion.”What he might not have guessed when he startedWe found when we co<strong>mb</strong>ined the herb with chemotherapy, the anti-tumor activity was maintained.In fact, [it seemed to be] enhanced. So in a new study [in patients undergoing chemotherapyfor metastatic colon cancer], we’re going to be looking at not only the effect on toxicity but alsoresponse rates, progression-free survival, overall survival, and quality-<strong>of</strong>-life <strong>issue</strong>s.We are trained, as Western physicians, to look for the active ingredient that can target a particularkey pathway involved in tumor growth and proliferation. In traditional Chinese medicine andEastern medicine, the idea is to use a formulation that’s made up <strong>of</strong> multiple components [like HIVdrug cocktails]. In animal studies, what we found is you needed all four herbs to be able to maximizethe anticancer therapy and to maximize the so-called cytoprotective activity.—Interview by Erica LloydCAMI MESAThis year, Brian Zuckerbraun and Nuria M.Pastor-Soler were invited to join theAmerican Society for Clinical Investigation(ASCI). The society, established in 1908, is one<strong>of</strong> the nation’s oldest and most respected medicalhonor societies. According to its Web site, it“represents active physician-scientists who areat the bedside, at the research bench, and at theblackboard.”Pastor-Soler, an assistant pr<strong>of</strong>essor <strong>of</strong>medicine and <strong>of</strong> cell biology, is investigatingthe regulation <strong>of</strong> a protein complex that pumpsprotons across me<strong>mb</strong>ranes, helpingto guide the normal function <strong>of</strong> kidneytubules and the male reproductive tract.Pastor-Soler’s work has led to significantfindings regarding the behavior <strong>of</strong> kidneyswhen acid levels in bodily fluid areabnormally high (acidotic states) as wellPastor-Soleras sperm maturation and male fertility.She believes that her lab’s work will alsohelp elucidate how kidney cancer cellsmetastasize.Zuckerbraun’s laboratory hasfocused primarily on the use <strong>of</strong> gaseoussignaling molecules and their protectiveeffects. He has investigated the useZuckerbraun<strong>of</strong> carbon monoxide and nitric oxide asshielding agents against organ and vascularinjury in a nu<strong>mb</strong>er <strong>of</strong> disease states. He says heis “very interested in developing therapeuticsthat harness the effects <strong>of</strong> these gaseous moleculesand signaling pathways to protect againstt<strong>issue</strong> injury and death.”Up to 80 individuals can be inducted eachyear—ASCI’s active me<strong>mb</strong>ers must first approvethe group—and only those who are under 45years <strong>of</strong> age are considered. <strong>Pitt</strong> med claims 22ASCI me<strong>mb</strong>ers.In addition, two School <strong>of</strong> <strong>Med</strong>icine facultyme<strong>mb</strong>ers were elected to the Association<strong>of</strong> American Physicians (AAP) this year.Neurological surgery chair Robert Friedlander,an MD and UPMC Endowed Pr<strong>of</strong>essor <strong>of</strong>Neurological Surgery, and Ian Pollack—MD,codirector <strong>of</strong> the Brain Tumor Program at the<strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh Cancer Institute, chief<strong>of</strong> pediatric neurosurgery at Children’s Hospital<strong>of</strong> <strong>Pitt</strong>sburgh <strong>of</strong> UPMC, and <strong>Pitt</strong>’s Walter DandyPr<strong>of</strong>essor <strong>of</strong> Neurosurgery—were among the 61named to join the elite, 127-year-old organization.—Hayavadhan Thuppal4 PITTMED
All in the FamilyThis summer, <strong>Pitt</strong>’s Department <strong>of</strong> Family<strong>Med</strong>icine, with UPMC and WesternPsychiatric Institute and Clinic (WPIC),graduated its second trainee from thejoint family medicine and psychiatry residencyprogram, which was established in2007 and is one <strong>of</strong> only a handful in thecountry. James Dewar, an MD, associatepr<strong>of</strong>essor <strong>of</strong> family medicine, and thedepartment’s vice chair for education,says the program addresses an underservedneed in family medicine. About 30to 40 percent <strong>of</strong> patients seen by familyphysicians show symptoms <strong>of</strong> mentaldisorders, he says. The joint program,says Michael Travis, MD and director <strong>of</strong>Psychiatry Residency Training at WPIC,teaches its graduates to excel as familyphysicians and psychiatrists. —JMCATHERINE LAZUREUNIVERSITY OF PITTSBURGH HISTORIC PHOTOGRAPHSFLASHBACKToday, the Falk <strong>Med</strong>ical Building is known as a hub<strong>of</strong> clinical care and is home to the General InfectiousDiseases Clinic and HIV/AIDS Care Center. But anotherkind <strong>of</strong> home occupied the space opposite MeyranAvenue at Fifth Avenue until construction <strong>of</strong> the FalkClinic, funded by brothers Maurice and Leon Falk, beganin the late 1920s. Instead <strong>of</strong> demolishing the privateresidence standing on the site, workers put it up on railsand moved it down the street to make room for the newoutpatient dispensary.EVOLUTION OF A DEPARTMENTPlastic surgery has long been one <strong>of</strong> the School <strong>of</strong> <strong>Med</strong>icine’sstrengths. On July 1, this strong division <strong>of</strong> the Department <strong>of</strong>Surgery became a department in and <strong>of</strong> itself.Founding chair <strong>of</strong> the Department <strong>of</strong> Plastic Surgery,J. Peter Rubin, an MD, says that coming out from under theu<strong>mb</strong>rella <strong>of</strong> the larger department is a logicalstep. “We’ve seen this [similar evolution] withorthopaedics, otolaryngology, and neurosurgery,”he says. “We have developed our own educationalpathway, separate board certification; we’vehad our own journal since the 1940s. Taken alltogether, plastic surgery is truly an independentspecialty.”The division’s ascension to department statusRubinwas celebrated in a ceremony in the CommonsRoom <strong>of</strong> the Cathedral <strong>of</strong> Learning on June 29 with a crowd<strong>of</strong> 275, including plastic surgery specialists from around thenation, 50 <strong>of</strong> them residency alumni. The new department has 19clinical and five research faculty with the rank <strong>of</strong> associatepr<strong>of</strong>essor or higher. —JM4 PITTMED FALL 2012 5
tessulatusammiralisgloriamarisA SNAIL’S TRACESea snails in the Conus genus are known for their beautiful shell patterns that vary fromspecies to species. These intricate patterns are created by the snail’s neural responses.The patterns amount to a visible record <strong>of</strong> the nervous system—an organ that can’t bepreserved in the fossil record. Earlier this year, researchers, including <strong>Pitt</strong>’s Distinguished<strong>University</strong> Pr<strong>of</strong>essor <strong>of</strong> Computational Biology G. Bard Ermentrout, announced they’d createda computer model capable <strong>of</strong> illustrating evolutionary changes in Conus pigmentationand used it to model living as well as ancestral species . The snails (and some heady computing)have given scientists a window to neural evolution. (In the above graphic, bracketsto the far left show modeled, ancestral shells. On the far right are actual, present-dayshells paired with their models.) —JHdallifurvustextilecrocatusomariaaulicusepiscopatusbandanusmarmoreuslaterculatuspulicariusconsorsarenatusaurisiacusstercusmuscarumorbignyiCOURTESY G.B. ERMENTROUTName DroppingThe <strong>University</strong>’s annual science festival reliablybrings a nu<strong>mb</strong>er <strong>of</strong> fascinating folks to campus.This year’s festival, Science2012—Translation,which kicks <strong>of</strong>f October 3 this year, features alecture by Brian Druker, winner <strong>of</strong> <strong>Pitt</strong>’sDickson Prize in <strong>Med</strong>icine. Druker, an oncologistmost known for his central role in the development<strong>of</strong> imatinib (Gleevec), a drug that targets themolecular defect in chronic myeloid leukemia, alsospearheaded the clinical trials <strong>of</strong> the drug. (It’s anapproved treatment for seven cancers.) He directsthe Knight Cancer Institute at Oregon Health &Science <strong>University</strong>, where he is also associate deanfor oncology, JELD-WEN Chair <strong>of</strong> Leukemia Research,and a Howard Hughes <strong>Med</strong>ical Institute (HHMI)Investigator. Among Druker’s litany <strong>of</strong> awards arethe American Cancer Society <strong>Med</strong>al <strong>of</strong> Honor, theCharles F. Kettering Prize from General MotorsCancer Research Foundation, the Lasker-DeBakeyClinical <strong>Med</strong>ical Research Award, and the JapanPrize in Healthcare and <strong>Med</strong>ical Technology. He isa me<strong>mb</strong>er <strong>of</strong> the National Academy <strong>of</strong> Sciences andthe American Academy <strong>of</strong> Arts and Sciences.Ronald R. Breaker, HHMIInvestigator and Henry Ford II Pr<strong>of</strong>essor and chair<strong>of</strong> the Department <strong>of</strong> Molecular, Cellular, andDevelopmental Biology at Yale <strong>University</strong>, willdeliver the 2012 Mellon Lecture. His work led to thediscovery and characterization <strong>of</strong> many new RNAs,including riboswitches—a mode <strong>of</strong> gene regulationin which metabolites regulate the activity <strong>of</strong> certainpathways by directly binding to messenger RNAwithout the direct involvement <strong>of</strong> proteins. Breaker’swork has been honored with the National Academy<strong>of</strong> Sciences’ Award in Molecular Biology.H<strong>of</strong>mann Lecturer Karl Deisseroth,an MD/PhD, led the way in the development andapplication <strong>of</strong> optogenetics, a technology that “useslight to control millisecond-precision activity patternsin genetically defined cell types within thebrains <strong>of</strong> freely moving mammals,” says Deisseroth.This method allows for the real-time study <strong>of</strong> therelationship between neural circuits and behavior.Deisseroth, associate pr<strong>of</strong>essor <strong>of</strong> bioengineeringand <strong>of</strong> psychiatry and behavioral sciences at Stanford<strong>University</strong> and an HHMI Early Career Scientist,received the 2010 Koetser Prize and the 2011W. Alden Spencer Award for his work. —JM6 PITTMED
CLOSER7/17/12CLOSER LAYOUTBACK TO YOU, SUSANAs a woman lay in front <strong>of</strong> her on <strong>Pitt</strong>sburgh’s South Negley Avenue,fourth-year medical student Vanessa Franco was faced with a prematureand unexpected test <strong>of</strong> her training.“Her face was pretty blue,” says Franco. “When I checked her pulse,I realized she didn’t have one.” A fellow fourth-year, Ranmal Samarasinghe,monitored the woman’s vital signs as Franco performed CPR chestcompressions. (<strong>Med</strong> students at <strong>Pitt</strong> are required to be certified in CPR,though neither Franco nor Samarasinghe had ever administered it beforeon anyone.)“I was terrified <strong>of</strong> losing her,” Franco revealed to a reporter later.Gradually, the color returned to the woman’s face, and paramedicsarrived with an AED to shock her heart back into rhythm before she wasrushed to UPMC Shadyside’s ER.The woman collapsed on a Sunday morning last Nove<strong>mb</strong>er, as Francoand Samarasinghe were driving to Giant Eagle in Shadyside. Where SouthNegley crosses the Port Authority East Busway, a group <strong>of</strong> people huddledaround the woman were yelling for help. Franco and Samarasinghe pulledover and intervened. It turned out that the woman was a KDKA newsanchor and mother <strong>of</strong> three, Susan Koeppen (shown above, flanked by thestudents), who suffered from a defect <strong>of</strong> the mitral valve, which facilitatesthe flow <strong>of</strong> oxygenated blood in the heart. She had gone into cardiac arrestwhile on a run with friends. (She was training for a half marathon.)In March, the now-40-year-old Koeppen underwent open-heart surgery torepair the faulty valve, then made a full recovery, and resumed her positionat the news desk in May.Samarasinghe and Franco are in the medical scientist training program;they will finish their MDs this fall and then pursue postdoctoral workbefore starting residencies. Both have PhDs in neuroscience. Franco hopesto enter emergency medicine; Samarasinghe recently decided on neurology.Franco has been pursuing research on marathon runners and hearthealth. She conducted a pilot study during the <strong>Pitt</strong>sburgh Marathon thatshe hopes to replicate in other races around the country. (She pursued thestudy with the help <strong>of</strong> executive vice chair <strong>of</strong> emergency medicine CliftonCallaway, an MD/PhD who is the Ronald D. Stewart Pr<strong>of</strong>essor <strong>of</strong> Emergency<strong>Med</strong>icine, and Dave Hostler, a PhD research associate pr<strong>of</strong>essor <strong>of</strong>emergency medicine.) The encounter with Koeppen has made the workeven more meaningful to Franco. —by Hayavadhan Thuppal—Photo by Martha RialSUMMER FALL 2012 75
INVESTIGATIONSExplorations and revelations taking place in the medical school8 PITTMEDLymphatic vessels (purple)—which drain lymphaticfluid from t<strong>issue</strong>—next to blood vessels (blue)
THE FAMILY THATASSAYS TOGETHERTHE GENETICS OF LYMPHEDEMA REVEALEDAT A REUNION | BY ALLISON A. CURLEYJENNY M. KARLSSON, CENTER FOR BIOLOGIC IMAGINGNearly two decades ago, <strong>Pitt</strong>’sDavid Finegold (BS ’68, MD’72, Res ’75), pr<strong>of</strong>essor <strong>of</strong>pediatrics and medicine, and Robert Ferrell,pr<strong>of</strong>essor <strong>of</strong> human genetics in the GraduateSchool <strong>of</strong> Public Health, were chatting overa cup <strong>of</strong> c<strong>of</strong>fee. Finegold mentioned thathis wife, physiatrist Judith Esman (Res ’87),worked with patients with lymphedema, an<strong>of</strong>ten-disabling retention <strong>of</strong> fluid (usually inthe li<strong>mb</strong>s) that results from abnormal drainage<strong>of</strong> the lymphatic system. Ever the curiousgeneticist, Ferrell asked his usual question:“Is it ever inherited?” As it happened, Esmanwas treating a father and his twin daughtersfor Milroy’s disease, a hereditary form <strong>of</strong>lymphedema. In fact, many me<strong>mb</strong>ers <strong>of</strong> thisfamily were affected, and they were willing toparticipate in research. Finegold and Ferrellcouldn’t pass up the opportunity to study anobscure disorder that was clearly genetic, andso they set out to identify the genes underlyingMilroy’s disease.Some days later, the researchers rentedout a local fire hall and hosted a familyreunion. At one end <strong>of</strong> the hall were cookiesand c<strong>of</strong>fee and at the other, blood drawsand clinical exams. The team collected DNAsamples from 40 family me<strong>mb</strong>ers; othersmailed in samples later. Such was the beginning<strong>of</strong> the largest-ever genetic study <strong>of</strong> heritablelymphedema, the <strong>Pitt</strong>sburgh LymphedemaFamily Study, which now includes more than300 families worldwide.Three years in, Finegold and Ferrell identifiedthe first-known causative gene, whichcodes for a protein named VEGFR3 and isimportant in the development <strong>of</strong> the lymphaticsystem. They went on to find threemore <strong>of</strong> the seven known causative genes—and have pinpointed the genetic culprits inabout a third <strong>of</strong> the families they’ve studied.The lymphatic system is a network <strong>of</strong> vesselsthat collect fluid from t<strong>issue</strong> throughoutthe body and deliver it to the bloodstream andlymph nodes. Hereditary (or primary) lymphedema,in which the system develops abnormally,occurs in roughly one in 6,000 people.Far more common is secondary lymphedema.Secondary lymphedema occurs in about30 percent <strong>of</strong> women treated for breast cancer;for many years, doctors assumed it was aresult <strong>of</strong> the trauma <strong>of</strong> surgery, chemotherapy,or radiation. But not everyone in treatmentgets lymphedema, so Ferrell and Finegoldreasoned that trauma probably isn’t the onlycause. They suspected that genes played a rolein this form <strong>of</strong> lymphedema, too, and set outto prove it. “If we’re right, and we can identifya subset <strong>of</strong> women who are going to be at risk,we can potentially start preemptive treatment,such as massage and compression garments,”says Finegold.In April, the team published results <strong>of</strong> astudy that examined several candidate genesin a population <strong>of</strong> women with secondarylymphedema following breast cancer treatment.They found that these women—butnot healthy women or those with breast cancerwho hadn’t experienced secondary lymphedema—hadmutations in the gene codingfor connexin 47 (Cx47). The researchers hadpreviously implicated the gene in primarylymphedema.Drawing on the expertise <strong>of</strong> research assistantpr<strong>of</strong>essor <strong>of</strong> cell biology Catherine Baty,who joined the team in 2008, the researchersalso examined the functional consequences<strong>of</strong> the Cx47 mutations. Connexins are theprimary components <strong>of</strong> structures called gapjunctions; recent evidence suggests they areimportant in the movement <strong>of</strong> lymph fluid.The investigators reasoned that the deficitsresulting from the mutations may be subtle,because patients don’t develop the conditionuntil after the insult <strong>of</strong> cancer treatment.Using cultured human cells with mutationsintroduced, they showed that proteins traffickednormally to the cell surface but exhibitedother functional impairments.Ultimately, the team hopes that knowledge<strong>of</strong> the underlying genes could lead to aSecondary lymphedema occurs in about 30 percent<strong>of</strong> women treated for breast cancer.cure. In fact, drugs modifying the function<strong>of</strong> connexins are already available; they wereinitially developed for cardiovascular disease.The findings could also have implicationsfor wound healing among other conditionslinked to fluid i<strong>mb</strong>alance, says Ferrell. Hecalls lymphedema a “window on lymphatics”and a way to identify genes that are importantin the functioning <strong>of</strong> the lymphatic system asa whole.FALL 2012 9
“CAPTAIN” GENEA NEW TARGETFOR FIBROID THERAPYBY DANA YATESJUST FOLLOWING ORDERSThe National Institutes <strong>of</strong> Healthreports that, by age 50, half <strong>of</strong>all women will have fibroids.Consisting <strong>of</strong> muscle cells and other t<strong>issue</strong>sfound in and around the wall <strong>of</strong> the uterus,fibroids cause no symptoms for some women.For others, however, fibroids can lead to arange <strong>of</strong> problems. They include frequenturination, lower back pain, heavy menstrualbleeding, painful sex, a sensation <strong>of</strong> fullness orpressure in the lower abdomen, miscarriages,and premature labor. In extreme cases, fibroidscan weigh up to 100 pounds, causing severedisfigurement. Fibroids are the leading cause <strong>of</strong>hysterectomies in the United States.But what causes them? A discovery madeby the <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh’s AleksandarRajkovic indicates that, for the majority <strong>of</strong>women, a single gene mutation is to blame.Rajkovic, an MD/PhD and associate pr<strong>of</strong>essorin the School <strong>of</strong> <strong>Med</strong>icine’s Department<strong>of</strong> Obstetrics, Gynecology, and ReproductiveSciences, is division chief <strong>of</strong> genetics atMagee-Womens Research Institute. He is alsothe senior author <strong>of</strong> a study that identifiedthe genetic pathway that contributes to thedevelopment <strong>of</strong> uterine fibroids. The team’sfindings were published in the online journalPLoS One in March.“Right now, the only treatment for fibroidsinvolves surgically removing them from theuterus. But that solution isn’t perfect,” he says.“For approximately 50 percent <strong>of</strong> women, thefibroids come back, and redoing the surgeryis associated with even more complications.”Among them, infection, loss <strong>of</strong> the wholeuterus, and death.As a gynecologist who is familiar withthose risks, Rajkovic is focused on findingnoninvasive ways to eliminate fibroids. Usinggenome-sequencing technology, Rajkovic’sgroup figured out the exact order <strong>of</strong> the chemicalbuilding blocks <strong>of</strong> fibroid and healthyuterine t<strong>issue</strong>s.“We wanted to know if there were differencesor genetic changes in the t<strong>issue</strong>s thatallowed fibroids to grow,” he says.By examining the fibroid and normal uterinet<strong>issue</strong>s from five women who had undergonehysterectomies, the research team learnedthat three <strong>of</strong> the women had fibroids withmutations in a gene called MED12. A regulatorygene, MED12 acts like a captain, tellingthe genes downstream what to do.The researchers broadened their explorationand turned to a biobank for additionalt<strong>issue</strong> samples, looking for the MED12 mutationin 143 uterine fibroids. The results:approximately 70 percent <strong>of</strong> the samples containedthe mutation, whereas normal uterineLEFT: Genome sequencing <strong>of</strong> normal uterinet<strong>issue</strong>. RIGHT: Highlighted letters show agenetic change on the X chromosome. A singlegene mutation, MED12, causes fibroid tumorgrowth—it was found in 70 percent <strong>of</strong> thefibroid samples Rajkovic studied.t<strong>issue</strong> samples did not.“Ultimately, we hope to target theother players that MED12 regulates,” saysRajkovic.“They are the major carriers <strong>of</strong> the informationthat MED12 is trying to convey,and we could modify their function pharmaceutically.”Rajkovic’s research group is now exploringthe mechanism between MED12 andother genes that take orders from it. To thatend, the researchers are working with micewith modified MED12 genes. The team alsowants to determine whether the MED12mutation makes fibroid regrowth more orless likely after surgical treatment.And what about the fibroid samples thatlack the MED12 mutation? In those cases,Rajkovic speculates, multiple genes maybe involved, interacting with environmentalfactors to spur fibroid growth. Futureresearch studies will investigate that process,but Rajkovic is still busy unraveling theMED12 mystery.RAJKOVIC LAB10 PITTMED
JUAN CARLOS ALVAREZ-PEREZBETA UPPregnant mice engineeredto lack the sensor for hepatocytegrowth factor (right)produce fewer beta cells,shown in pink, than normalpregnant mice do (left).CAN HGF STRIKE OUT GESTATIONAL DIABETES?BY MELINDA WENNER MOYERFor an expectant mother, a diagnosis<strong>of</strong> gestational diabetes—whichafflicts 135,000 pregnant womenin the United States every year—can be terrifying.For the remainder <strong>of</strong> her pregnancy,she must carefully monitor her blood sugaras well as her baby’s size to minimize the risk<strong>of</strong> labor difficulties and stillbirth. Further, sheand her baby will always be at heightened riskfor type 2 diabetes. Historically, researchershave understood very little about the cause <strong>of</strong>gestational diabetes, but a new study by a teamled by Adolfo Garcia-Ocaña, a PhD associatepr<strong>of</strong>essor <strong>of</strong> medicine at the <strong>University</strong><strong>of</strong> <strong>Pitt</strong>sburgh, suggests that the differencebetween a healthy pregnancy and a diabeticone may boil down to problems with a singleliver protein and its molecular sensor.This protein, called hepatocyte growthfactor (HGF), has actually been a longtimeinterest <strong>of</strong> Garcia-Ocaña’s. It was discoveredin 1990 by <strong>Pitt</strong>’s George Michalopoulos,MD/PhD and chair <strong>of</strong> <strong>Pitt</strong>’s Department <strong>of</strong>Pathology. Soon after arriving at <strong>Pitt</strong> in 1997,Garcia-Ocaña wondered: Given HGF’s abilityto induce cellular growth in t<strong>issue</strong>, could theprotein be used to grow more beta cells (foundin the pancreas) in people with type 1 and type2 diabetes—diseases characterized in part by apaucity <strong>of</strong> beta cells? In 2000, Garcia-Ocañagenetically engineered mice so that their betacells over-produced HGF, and the mice grewmore and bigger beta cells than normal micedid. HGF, it seemed, played an important rolein beta-cell growth and replication.It occurred to Garcia-Ocaña that betacellgrowth was also an important aspect <strong>of</strong>a healthy pregnancy. “A mother adapts toenhanced metabolism during pregnancy ina way that increases the nu<strong>mb</strong>er <strong>of</strong> insulinproducingcells and increases the amount <strong>of</strong>insulin that’s being produced by these cells,” heexplains. After delivery, her body kills <strong>of</strong>f theextra beta cells. He knew from research publishedin 1995 that during pregnancy, HGFlevels in the body skyrocket. “So we thought,Hmmm. Maybe HGF plays a role in gestationaldiabetes.” Perhaps the condition arises whenHGF is unable to do its job, he imagined.In order for pancreatic cells to respond toHGF, they have to express its sensor, c-MET,on their surface. Garcia-Ocaña wondered whatwould happen if HGF couldn’t communicatewith c-MET during pregnancy. To find out,he genetically engineered female mice to beunable to produce c-MET on their beta cells.Then he watched what happened.At first, the mice did just fine. They seemedperfectly normal. But then, when they becamepregnant, “the expansion <strong>of</strong> insulin-producingcells was blunted, and insulin secretion wasalso diminished,” he explains.In other words, “These mice developedgestational diabetes.”When Garcia-Ocaña studied the mice further,he discovered that their bodies prematurelykilled <strong>of</strong>f beta cells during late pregnancy,too.Garcia-Ocaña and his collaborators—whoinclude the School <strong>of</strong> <strong>Med</strong>icine’s Cem Demirci,Sara Ernst, Juan Carlos Alvarez-Perez, andGabriella Casinelli, among others—publishedthe findings online in March in Diabetes.He doesn’t yet know whether women whodevelop gestational diabetes tend to have problemswith HGF or c-MET or both. So histeam will analyze blood samples from thispopulation to find out more.Another unanswered question is whetherHGF and c-MET affect beta-cell growthdirectly, or do so by way <strong>of</strong> additional proteinsor receptors.Garcia-Ocaña wonders whether doctorsmight one day treat or prevent gestationaldiabetes with therapies that regulate abnormalHGF/c-MET signaling.He’s considering the implications for type1 or 2 diabetes, as well. Boosting the growth<strong>of</strong> insulin-producing beta cells might treat orprevent those disorders, too.FALL 2012 11
12 PITTMED
FEATURENOT QUITE CATCHING UP WITHROBERT FRIEDLANDERBY DAVID R. ELTZEVERYMOVEMENT COUNTSRobert Friedlander is, among other things, a neurosurgeon.Whenever he is working inside someone’s brain,excising a brain tumor or fixing an artery, he is in astate <strong>of</strong> heightened awareness. In his line <strong>of</strong> work, wandering just amillimeter <strong>of</strong>f could mean nicking a nerve, clipping a blood vessel,altering lives. “Every movement counts,” says Friedlander, who is chair<strong>of</strong> the <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh’s Department <strong>of</strong> Neurological Surgery.That philosophy seems to apply to all aspects <strong>of</strong> his life. Friedlanderhas made every movement count in a career in which he has not onlyhelped patients with his surgical talents, but also made important discoveriesabout programmed cell death and how interrupting that cascade<strong>of</strong> death may one day translate into treatments for patients withneurological diseases.Growing up in Caracas, Venezuela, Friedlander knew early that hewanted to be a doctor and a scientist. In high school, he read an influentialScientifi c American paper on oncogenes, which convert normal cellsinto cancer cells, by MIT’s Robert A. Weinberg. Yet, higher educationRobert Friedlanderis thought to be thefirst neurosurgeon todo research on celldeathpathways. PHOTOGRAPH | JIM JUDKISFALL 2012 13
wasn’t a tradition in his immediate family.His Jewish grandfather had escaped fromPinsk, Russia, after half his family was murderedin pogroms. At 15, his grandfather fledwith the equivalent <strong>of</strong> $30 in his pocket andmade his way to Cuba, eventually ending upin Venezuela with a wife from Brooklyn viaan arranged marriage that would produceFriedlander’s mother. Friedlander’s paternalgrandparents had escaped the Nazis prior toWorld War II to what would become Israel.His father eventually visited Venezuela, wherehe met Friedlander’s mother at a high schooldance and stayed to marry her.Friedlander’s parents encouraged their sonto pursue his dreams. Two <strong>of</strong> Friedlander’suncles had left Venezuela to become doctors inBoston. When he was just out <strong>of</strong> high school,Friedlander applied to Brandeis <strong>University</strong>near Boston, figuring an American educationwould give him the best opportunities. “That’swhere things happen,” he says.He was wait-listed.Friedlander had learned to speak Englishfrom his grandmother. But realizing he neededto improve his writing and reading skills inthe language to get into Brandeis, he took asix-month English course in Boston. Oncefinished, he was accepted into Brandeis andenrolled in an a<strong>mb</strong>itious dual bachelor’s andmaster’s program in biochemistry. Friedlandersoon discovered he needed to improve inother areas. His first test was in calculus; hegot a C+.“I said, Hmmm … that’s not going to getme into medical school. It was a wake-up call,”says Friedlander, who buckled down, pushinghimself like never before.Between 1985 and 1987, Friedlanderworked in the lab <strong>of</strong> Michael Newman, a PhDand an assistant pr<strong>of</strong>essor <strong>of</strong> biochemistry. InNewman’s lab, Friedlander learned to growcells in t<strong>issue</strong> culture to try to understand howa normal cell became a cancer cell.“He was always coming back to me andmaking suggestions and going above andbeyond what the typical undergraduate woulddo in pursuing research,” says Newman, nowexecutive vice president <strong>of</strong> research and developmentat Vaxiion Therapeutics in San Diego.Friedlander finished both his bachelor’s andmaster’s degrees in three-and-a-half years—while working with Newman and attendingclasses every summer in Boston—and wasaccepted into Harvard <strong>Med</strong>ical School. AtHarvard, Friedlander wanted to continue todo research. Initially, having read Weinberg’spaper on oncogenes years earlier, he was interestedin studying cancer. (Cancer had run inFriedlander’s family.) But he also recognizedthat the fields <strong>of</strong> genomics and cell biologywere taking <strong>of</strong>f.When Friedlander started rotations duringhis third year <strong>of</strong> medical school, he considereda surgical subspecialty, but the grueling hoursand years <strong>of</strong> training required made himhesitate. One <strong>of</strong> his mentors, Charles McCabe,then the director <strong>of</strong> the surgical clerkship atHarvard, told Friedlander a parable aboutwhat could happen if he chose an easier pathin medicine.McCabe said: You could choose somethingelse and have a good lifestyle, but if you don’tlike what you’re doing in your practice, you’regoing to hate waking up. You’re going to hategoing to work. You’re going to come home maybeat 5 o’clock and be in a bad mood. And you’renot going to be nice to your wife and your kids.Or, McCabe told Friedlander, he couldpursue training that might take two or threeyears longer. He would have the rest <strong>of</strong> hislife to become the kind <strong>of</strong> physician andresearcher he wanted to be. And he’d be doingwhat he loved.Friedlander took his mentor’s advice. Hegraduated from med school in 1991 andstarted a seven-year residency in neurosurgeryat Massachusetts General Hospital. He workedthrough his residency, contemplating whetherto become either a neurovascular or braintumor surgeon. Then, something else caughthis attention. During a neurosurgeon updatecourse, Friedlander attended a talk by MITpr<strong>of</strong>essor H. Robert Horvitz, the founder <strong>of</strong>the emerging field <strong>of</strong> cell death.“It was one <strong>of</strong> those lectures ... where yourheart starts beating really fast, and you’re reallyexcited about something. Something that all<strong>of</strong> a sudden opens your eyes,” Friedlander says.Friedlander was hooked by Horvitz’s science.After the talk, he approached him to askhow he could research cell death. Horvitz puthim in touch with Junying Yuan, an assistantpr<strong>of</strong>essor at Harvard who at the time had aresearch lab in Massachusetts General. Yuanhad discovered the caspase 1 pathway (a dyingcell’s executioner)—the first insight into themolecular mechanism that regulates apoptosis(programmed cell death), which is crucial tomany biological processes, including e<strong>mb</strong>ryogenesis.(Horvitz, who earned a share <strong>of</strong> the NobelPrize in Physiology or <strong>Med</strong>icine in 2002, hascredited Yuan with doing a third <strong>of</strong> the work thatearned him the award.)Yuan took Friedlander into her lab in 1994.Their first goal was to understand the basicmechanisms <strong>of</strong> cell death.“When Robert first came on board, he wasjust a fellow who had never done any research[like this] before,” says Yuan, now a pr<strong>of</strong>essor<strong>of</strong> cell biology at Harvard. “I was really worriedabout him because most people in my lab weremuch more advanced in terms <strong>of</strong> science.”But Friedlander caught on fast. At the time,scant evidence implicated activation <strong>of</strong> the caspasecell-death pathway in neurological diseases.Figuring he was eventually going to become astroke researcher, Friedlander, with Yuan, createda transgenic mouse model (it made a protein thatblocked cell death in the brain and spinal cord);then he made the mouse have a stroke. When hedid, he saw that caspase was indeed activated inneurological disease. He also discovered that ifhe blocked the pathway with a drug, he couldprotect the mouse from suffering massive celldeath during a stroke and shield the mouse fromHe had planned to become a stroke researcher, but all<strong>of</strong> a sudden he was a famous ALS researcher.neurological damage.Working from that model, Friedlander beganto think about inhibiting cell death in amyotrophiclateral sclerosis (ALS), or Lou Gehrig’sdisease. Other researchers had created a mouse tostudy ALS using the mutant SOD1 gene, whichhad been identified as a cause <strong>of</strong> ALS. If thegene overexpresses its protein, the mouse’s motorneurons would die, and the mouse would endup with progressive neurodegeneration, then die.This disease course is similar to what happens inhumans with familial ALS. Friedlander took hiscell-death-resistant mouse and crossed it with theALS mouse. The <strong>of</strong>fspring lived longer. This wasthe first time a scientist had been able to extendthe life <strong>of</strong> an ALS mouse. The results were publishedin Nature on July 3, 1997.But the research part <strong>of</strong> his residency wasending. Yuan was moving her lab to Harvard,and he’d have to return to the hospital staff. YetFriedlander wasn’t about to give up on his studies.So he secured a small grant from the MuscularDystrophy Association and his own small lab to14 PITTMED
continue the studies while going back to a rigoroushouse staff schedule. Friedlander was able toshow that blocking the gene coding for a proteincalled ICE in the caspase 1 pathway in an ALSmouse slowed progression <strong>of</strong> the disease, andthe mouse lived longer after getting the disease.Here was another crossroads. He was tryingto finish his residency and to become a strokeresearcher, but all <strong>of</strong> a sudden he was a famousALS researcher. “That was not the plan,” he says.“But if you see an opportunity, you take it.”Around the same time, other researchers hadcreated a transgenic mouse to study cell death inHuntington’s disease, which affects muscle coordinationand leads to cognitive decline, psychiatricproblems, and eventual death. Nancy Wexler,president <strong>of</strong> the Hereditary Disease Foundation,had seen Friedlander’s Nature paper on ALS.She had a representative from the foundationgive him a call, asking, “Would you like to doresearch on Huntington’s?”Years earlier, Wexler had started researchnear Lake Maracaibo, a brackish body <strong>of</strong> waterin the eastern part <strong>of</strong> Venezuela, home to theworld’s largest concentration <strong>of</strong> people withHuntington’s disease. Wexler eventually discoveredthe gene that causes Huntington’s disease,and her work led to the creation <strong>of</strong> a genetic testfor the disease.The foundation gave Friedlander his firstbig grant. Meanwhile, the chair <strong>of</strong> Harvard’sDepartment <strong>of</strong> Neurosurgery <strong>of</strong>fered him atiny lab, “with the ceiling falling <strong>of</strong>f,” andFriedlander started research on Huntington’sdisease. By then, he was the chief resident atMassachusetts General Hospital.His Huntington’s research produced resultssimilar to those he’d found in ALS: The caspase1 cell-death pathway was activated inHuntington’s disease. Blocking the pathwaywhen breeding the Huntington’s mouse withthe model Friedlander had created (that inhibitedcell death in the brain) made the <strong>of</strong>fspringlive longer. So did delivering a caspase inhibitordrug to the brain. The results were published inNature. Here again, Friedlander got a diseasedmouse model to live longer when no one elsecould.After completing his residency in 1998 andtaking a position at Brigham and Women’s,Friedlander went on to use mice to make thefirst findings that the caspase family <strong>of</strong> cell-deathpathways was activated in many neurologicaldiseases, as well as in head trauma and spinalcord injury.Around the same time, other researchershad used minocycline—an antibiotic usedfor decades to treatacne and rheumatoidarthritis—toevaluate stroke.Friedlander wantedto see how the drugworked in his mice.He tried it in micewith traumatic braininjury, then his ALSmice, and finally hisHuntington’s mice.In each case, the animalswere protectedfrom damage andlived longer.He would eventually show that caspasesdon’t just abruptly kill <strong>of</strong>f a damaged cell.Their activity in one cell is essentially contagious,prompting the secretion <strong>of</strong> toxicsubstances that make neighboring neuronstoo sick to work properly.Despite his success at the bench,Friedlander hasn’t yet been able to demonstratethe same results in clinical trials. He hasworked with others to develop drugs to blockthe caspase family <strong>of</strong> cell-death pathways, yetnone <strong>of</strong> the drugs has worked in humans.Friedlander says part <strong>of</strong> the problem isin the challenge <strong>of</strong> translating research frommice into humans. Although disease in micemimics disease in humans, transgenic miceare essentially identical; they have the samedisease, the same mutation. Humans, however,are not all the same. Mutations and injuriesin humans vary from person to person.Friedlander, who became chair <strong>of</strong> theneurological surgery department at <strong>Pitt</strong> inJune 2010, has since been looking for otherdrugs that might work better than minocycline.He is collaborating with RobertFerrante, pr<strong>of</strong>essor <strong>of</strong> neurological surgery,whom Friedlander recruited to <strong>Pitt</strong> last yearfrom Boston <strong>University</strong>.In recent years, both Friedlander andFerrante have started to think about changingthe way clinical research is done. They believeresearchers should stop trying to see whetherjust one therapy can help patients and considerdrug cocktails instead. “Much <strong>of</strong> the targetcentricapproach that science has taken overthe past 50 years really hasn’t worked, becausewe’re only homing in on one particular aspect<strong>of</strong> a disease,” says Ferrante. “The currentapproach is to look at polytherapies. … We’refinally taking that approach to patients withchronic neurodegenerative disorders.”AMERICO TORRES/AFP/GETTY IMAGESVenezuela’s Lake Maracaibo area is home to the world’s largest concentration<strong>of</strong> people with Huntington’s disease.Friedlander is currently the only neurosurgeonon the advisory council <strong>of</strong>the National Institute <strong>of</strong> NeurologicalDisorders and Stroke. He’s also a recipient<strong>of</strong> the Society <strong>of</strong> Neurological Surgeons’ H.Richard Winn MD Prize, one <strong>of</strong> many honorsrecognizing his contribution to scienceand medicine. Her Royal Highness CrownPrincess Katherine <strong>of</strong> Serbia, who is knownfor her humanitarian projects, says, “there’snot enough recognition in the world” forFriedlander.Friedlander and his wife, Eugenia, metthe princess about 11 years ago. Since then,Princess Katherine, who helps people in hercountry get medical care, <strong>of</strong>ten calls on himto assist with patient cases: “I think he wasborn to save lives,” she says. “Every day <strong>of</strong> ourlives we are grateful to God that we have gonethrough this life and not missed meeting him.”Friedlander, so aware <strong>of</strong> making the most<strong>of</strong> his time, says <strong>Pitt</strong> has everything he waslooking for in a chair opportunity: visionaryinstitutional support from the medical schooland medical center, a vibrant neurosciencecommunity, an institute dedicated to academicdrug development, and the largest andbusiest neurosurgery department in the country.<strong>Pitt</strong> is also the home <strong>of</strong> many innovations,including transnasal (entering through thenose rather than opening the skull) endoscopicskull-based procedures, the Gamma Knife,microvascular decompression, and—amongthe latest—high-defi nition fi ber tracking,which is an enhanced MRI technique that forthe first time allows doctors to see connectivity<strong>of</strong> brain areas in 3-D (see cover story). Andhis department has been recognized for itsunusual productivity in basic science.“The opportunity here is once in a lifetime,”says Friedlander.FALL 2012 15
16 PITTMEDWith its million-fiber map,HDFT brings the wiry connections<strong>of</strong> the brain intobetter focus than everbefore. Here, a healthybrain shows no tracts aredisrupted.
COVER FEATURE STORYA NEW TECHNOLOGY ALLOWS DOCS TOSEE MISSING CONNECTIONS IN THE BRAINBY JENELLE PIFERMISSINGLINKSImagine you’re talking to a coworker about what you’d like toeat for lunch—a salad. You can see the salad in your mind—thegreens, the cherry tomatoes, the dressing on the side. You canvisualize the word, anticipate the consonants rolling around in yourmouth. You go to say it aloud—salad. But the word you know, cansee, can feel, can practically taste, escapes you. What’s that thing calledagain? You start to feel like you’re losing it.This was one symptom presented by a recent stroke patient seen byJuan Fernandez-Miranda, an MD and assistant pr<strong>of</strong>essor <strong>of</strong> neurologicalsurgery at the <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh and director <strong>of</strong> <strong>Pitt</strong>’s SurgicalNeuroanatomy Lab. “He has expressive aphasia,” says Fernandez-Miranda. “He has trouble articulating words.”The patient underwent an MRI, which confirmed the damage was concentratedin the left, language-storing side <strong>of</strong> the brain. “Some <strong>of</strong> the areas<strong>of</strong> the left hemisphere are smaller than normal,” says Fernandez-Miranda.But that’s really all the MRI could reveal—a thinning <strong>of</strong> white matter.What the picture didn’t show was the nature <strong>of</strong> the damage: which connectionswere broken and what exactly was blocking the word’s passagefrom brain to mouth. “Language is a dynamic process, so you need tounderstand the connectivity between areas to understand the problem.”A new technology developed at <strong>Pitt</strong> is making that connectivity clearfor the first time.IMAGES | SCHNEIDER LABFALL 2012 17
High-definition fiber tracking (HDFT)runs data from a top-end MRI machinethrough computational s<strong>of</strong>tware so that,instead <strong>of</strong> producing a flat, black-and-whitescan <strong>of</strong> t<strong>issue</strong>, it generates intricate 3-D images<strong>of</strong> connective fibers in the brain. Formerly,scientists could only study these connectionsduring post-mortem dissection, or in vaguedetail through less-precise scans. Now, brainconnectivity can be measured, much morethoroughly and accurately, in live patients.“In warfare and in medicine, it’s hard todefeat an enemy you cannot see,” says <strong>Pitt</strong> pr<strong>of</strong>essor<strong>of</strong> psychology and neurosurgery WalterSchneider, a PhD, whose team developed thetechnique. “That doesn’t mean you don’t tryand do things, but it’s certainly quite difficult.”Doctors and neuroscientists around theworld might use a scanning technique calleddiffusion tensor imaging (DTI), which measuresthe movement <strong>of</strong> water through thebrain. The idea is that by watching the waywater flows, you can determine the shapes <strong>of</strong>the fibers it runs through.The problem lies in the mathematics: DTIs<strong>of</strong>tware can’t account for instances where thefibers cross. And fibers cross all the time. Sowhen the technology detects water movingin one direction and then another, it simplytakes the average. The resulting scans show themajor stems <strong>of</strong> fiber tracts—a valuable and revolutionarybreakthrough—but the details arestill hazy and can lead to inaccurate diagnoses.“A fuzzy image doesn’t help you any clinically,”says Schneider, also a senior scientistin <strong>Pitt</strong>’s Learning Research and DevelopmentCenter. “[DTI] had the right intent but wasn’tpowerful enough to accomplish the goals thatwere intended.”Doctors need a technology that can followa fiber through its crossings, so five years agoSchneider and Sudhir Pathak, a programmerin his lab, set about refining the math. They’dalter the computational method, scan a brain,and show the image to Fernandez-Miranda,one <strong>of</strong> the world’s preeminent neuroanatomists.“He’d say, ‘Well, that’s good, but it’swrong,’” recalls Schneider.Fernandez-Miranda knew enough aboutfiber tracts from studying dissections to knowwhen the maps were <strong>of</strong>f. Year after year themethod got better. “Juan would say, ‘Hey, yougot this one right. How about this one?’” saysSchneider. By Septe<strong>mb</strong>er 2010, the techniquewas so precise it could out-predict Fernandez-Miranda. “We could noninvasively get fibertracts that were better than what he could dowith cadaver t<strong>issue</strong>.“At that point we hit a critical point <strong>of</strong> utilityand quality, and a lot <strong>of</strong> the clinical workbegan,” Schneider says.Everyone has the same 40 major fibertracts, connecting certain parts <strong>of</strong> the brain toothers, but—like the way arms and eyes differin size and shape—the way each tract’s fibersconnect can vary enormously. So studyingcadavers could only go so far in helping doctorsunderstand what might be happening intheir own live patients.Now the Schneider lab partners with ateam <strong>of</strong> basic scientists (researching things likevision, language, and attention), as well as ateam <strong>of</strong> 10 neurosurgeons who are applyingHDFT in the clinic to help people with conditionsas diverse as traumatic brain injuries,tumors, autism, and Alzheimer’s. More than120 patients have been scanned so far.“For surgery, the application is much moreimmediate,” says Fernandez-Miranda. A recentpatient, for example, presented with a tumorin the left corticospinal tract, which controlsmovement. A traditional MRI clearly revealedthe location <strong>of</strong> the tumor, but not what surroundedit.“I have a tumor here,” says Fernandez-Miranda, pointing to a mass on an MRI. “Butdo I have motor fibers here, or not? How aboutinside the tumor?” HDFT revealed that only afew fibers passed through the tumor and thatmost <strong>of</strong> the important ones had been pushedtoward the outside <strong>of</strong> the skull. Knowing this,they determined that the best approach wasfrom the midline.In presurgical planning, HDFT can alsopresent patients with the pr<strong>of</strong>ound opportunityto choose which side effects they’d preferto deal with. A surgeon can say, for example: Ifwe approach this way, we risk losing mobility;if we go in that way, we might compromisemood control. “Now we have a basis fromwhich to have a meaningful discussion withthe patient,” says Schneider.One <strong>of</strong> Schneider’s primary partnerships at<strong>Pitt</strong> is with David Okonkwo, an MD/PhD,associate pr<strong>of</strong>essor <strong>of</strong> neurological surgery, andclinical director <strong>of</strong> the Brain Trauma ResearchCenter. They are using HDFT to guide treatment<strong>of</strong> traumatic brain injury. In the UnitedStates, TBI occurs in 1.7 million people annuallyand is the most common cause <strong>of</strong> deathbetween ages 2 and 35. In most TBI cases,an MRI doesn’t show doctors much—maybesome swelling or bleeding, but not the actualdamage. This leads to a diagnosis that doesn’ttell patients, with much certainty, what’s wrongor how they can expect to heal.“Having an a<strong>mb</strong>iguous diagnosis is reallyproblematic,” says Schneider.“It’s okay to say, ‘You have swelling in yourhead. It’s a head cold, and it’ll clear up in threedays.’ But not to say, ‘We don’t know, you maynever work again in your life.’”Because doctors can’t see what’s broken inthe brain, they rely on outward manifestations—thesymptoms—to determine therapy.Recently a patient with TBI (who’d flipped hisATV, end-over-end) presented with limited18 PITTMED
With HDFT, fiber loss can beidentified and quantified bycomparing the injury site to thehealthy tract on the other side <strong>of</strong>the brain. Here, the right coronaradiata has sustained substantialfiber loss; the left side isuninjured.FALL 2012 19
mobility in his arm, hand, and leg. Traditionally he would have started intensivephysical therapy in each area, in the blind hope <strong>of</strong> regaining function. But withHDFT, his doctors can be more realistic, and strategic.By mapping what percentage <strong>of</strong> a given fiber tract is damaged, Schneider candetermine whether or not function is likely to return. For the patient in question,HDFT revealed projection to the hand was 97 percent lost, to the arm 67percent, and to the leg 60 percent. Although he could still move his arm, theman’s club hand would likely never heal. “People can get over losing a li<strong>mb</strong>: Theyunderstand it. They grieve the visible loss. They move on,” Schneider says. “Youdo nobody any good to tell them to work hard at something that’s impossible.We need to know how much is left so we know how best to invest hard butlimited hours <strong>of</strong> rehabilitation to bring back function.”The good news: Even though the patient couldn’t move his leg at the time,Schneider knew he had enough fibers remaining to walk again. “That’s going totake months <strong>of</strong> hard physical therapy,” Schneider says. “We would tell a patient,‘It’s not going to be easy, but we have scientific evidence that suggests it’s goingto come back. It’s still okay to work on the hand but concentrate on the leg.’”The technology can monitor how well a particular therapy works by keepingtrack <strong>of</strong> which fibers regrow. It’s probably not possible to grow a new fiber if allthe fibers <strong>of</strong> a tract are gone, Schneider says, but it is possible to increase the size<strong>of</strong> an existing fiber. “If you have a dirt road, we can make it into a highway. Ifyou don’t have the dirt road, we can’t.”In the case <strong>of</strong> Fernandez-Miranda’s stroke patient mentioned at the start—he’ll likely begin language therapy similar to what would have been prescribedwithout HDFT. But with HDFT, his doctor can see, in detail, whether thetherapy is changing anatomy. “You can first understand better the disease,” saysFernandez-Miranda. “By doing that, eventually, I think we’ll be able to designbetter therapies.”While HDFT is more accurate than any other technique available, there arestill blind spots. “We can detect a 20 percent loss <strong>of</strong> a tract, but it has to be a reasonablesize tract,” Schneider says. There are some tracts where if you lose 1 millimeter,you’ll never come out <strong>of</strong> a coma. “We aren’t that good yet,” Schneider says.“We’ve made enormous improvements in the last three years, but we certainlyhaven’t run out <strong>of</strong> ideas.” His goal, Schneider says, is to make what his teamdeveloped 18 months ago obsolete.20 PITTMED
THREADS OF LANGUAGEAND A VIEW OF TEMPLEGRANDIN’S BRAINThe left arcuate tract connects areas <strong>of</strong> the brain responsiblefor understanding language and expressing it. The damagedtract <strong>of</strong> a stroke patient (right) is visibly thinner than thenormal one (left), which explains why the patient can find theright word but can’t say it.As news spread about Walter Schneider’s HDFTtechnology, autism activist Temple Grandincaught word. She wanted to have her brainscanned. Until that point, the new and immenselyprecise scanning technology had not been usedto examine the brain <strong>of</strong> anyone with autism. “Wewere able to look at what might be different interms <strong>of</strong> her fiber tracts,” Schneider, <strong>Pitt</strong> pr<strong>of</strong>essor<strong>of</strong> psychology and neurosurgery, says.There are three subclasses <strong>of</strong> people withautism: those who never learn a language systemat all; those who repeat what they hear withoutunderstanding meaning; and those who are capable<strong>of</strong> language but at times have a hard time verbalizing,even though they know what they wantto say. Grandin is among the latter. (Grandin, whohas a PhD in animal science, now lectures widelyon autism as well as animal behavior and haswritten six popular books.)Upon reading the scan, Schneider saw thatfibers in Grandin’s motor system—in regionsthought to control repeating what you hear andnaming what you see—were barely there: Thetract was more than 90 percent smaller than acontrol subject’s. “I told Temple, ‘Your languagemay have been hanging on by a thread,’” saysSchneider. “It was enough, with intense training,to grow it.”Yet Grandin had four times as many fibersconnecting her visual system and frontal cortex,“potentially supporting her high visualizationabilities,” says Schneider.The two hope to partner on a project to mapthe brains <strong>of</strong> people in all three subclasses. S<strong>of</strong>ar only four patients with autism have beenscanned using HDFT.Typically, parents notice symptoms <strong>of</strong> autismwhen a child is between 18 months and 2 years <strong>of</strong>age. “The highest priority should be to establisheffective communication as soon as possible,”says Schneider, whether that communication isverbal, pointing, or otherwise, to minimize psychologicaldamage and stimulate language andcognitive abilities. HDFT can help decide whichmethod to choose.“The prize is to get a very early diagnosisso that you can speed the development <strong>of</strong> functionalcapability to be much, much earlier,” saysSchneider. —JPFALL 2012 21
22 PITTMED
FEATURETHE MITOCHRONICLES: PART 2BY JOE MIKSCHMITOCHONDRIA’SMANY MISSIONSIn the fi rst half <strong>of</strong> this series, scientists were going gaga over thelikely role <strong>of</strong> mitochondria, a long-neglected organelle, in cancer.<strong>Pitt</strong> mitochondriacs are also tackling Parkinson’s disease, hearthealth, and a devastating childhood disease. Here, you’ll readstories <strong>of</strong> resurrection, vital dances, and very sick fl ies.RUBEN DAGDA AND CHARLEEN CHUMe<strong>mb</strong>ers <strong>of</strong> CharleenChu’s lab have found thatmutations that causeParkinson’s diseasedecrease the nu<strong>mb</strong>er <strong>of</strong>mitochondria (green) indendrites (red), which arevital for receiving signalsin the brain. This imageshows mitochondria inhealthy neurons.The addicts <strong>of</strong> America needed a fix. War in poppy-producingAfghanistan and Turkey made it tough to get the realstuff. In 1976, a graduate student in chemistry, a Marylandman named Barry Kidston, tinkered around in his home lab and mixedup a super-potent synthetic called MPPP.The drug had been around since the 1940s and, back then, had 70percent <strong>of</strong> the potency <strong>of</strong> morphine. Through Kidston’s labors, he madeit even stronger and, therefore, all the more desirable to addicts. Then,in 1977, the 23-year-old injected some <strong>of</strong> his “home brew” as he <strong>of</strong>tenhad in the past. But something went wrong. Within three days he developedsymptoms <strong>of</strong> Parkinson’s disease.In 1982, 42-year-old George Carillo arrived at a hospital in San Jose,Calif. He was bent, twisted, drooling, and mute. His muscles were frozen.Six similar cases followed. Doctors became detectives. All sevenParkinsonian “frozen addicts” were found to have used a synthetic opiatecalled China White.FALL 2012 23
Then, a toxicologist involved in theCalifornia case recalled Kidston’s misadventure.Though it wasn’t big news at the time,authorities who examined Kidston’s lab founda very nasty impurity—MPTP—in the batchthat had rendered him ill. Six years later, thatsame impurity was found in the garage-labmadeCalifornia China White.And thus began an explosion in Parkinson’sresearch. Investigators found that when thebrain metabolizes MPTP, the byproductsinclude a toxin called MPP+. This MPP+,they eventually learned, attacks mitochondria—especiallythe beginning <strong>of</strong> the electrontransport chain, what’s known as complex I.This group <strong>of</strong> enzymes is associated with themitochondria’s best-known role: producingenergy for the cell. Later, researchers woulddiscover that complex I is inhibited in all cases<strong>of</strong> Parkinson’s, even in the rare inherited form.As the disease progresses, mito dysfunctionkills <strong>of</strong>f the dopamine-producing neurons inthe substantia nigra, a part <strong>of</strong> the brain thatcontrols voluntary movement.Enter <strong>Pitt</strong>sburgh’s mitochondriacs.The <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh’s J. TimothyGreenamyre—MD/PhD UPMC EndowedPr<strong>of</strong>essor <strong>of</strong> Neurology, chief <strong>of</strong> MovementDisorders, and director <strong>of</strong> both the <strong>Pitt</strong>sburghInstitute for Neurodegenerative Diseases(PIND) and the American Parkinson DiseaseAssociation Advanced Center for Parkinson’sDisease Research at <strong>Pitt</strong>—was among thoseintrigued by the relationship between toxins,mitochondria, and Parkinson’s.“In the ’80s, mitochondria research was abackwater,” says Greenamyre, whose investigations<strong>of</strong> the organelle stretch back to thatdecade. “People thought they knew everythingthere was to know—that mitochondria makeATP [fuel for cells]. It’s turned out to be muchmore complicated than that.”In the late 1990s, while he was on thefaculty at Emory <strong>University</strong> in Atlanta,Greenamyre began thinking that it mightbe useful to create a better animal model <strong>of</strong>Parkinson’s. He had a particular interest in replicatingthe manner in which damaged mitochondriacreate conditions that make neuronsinteracting with dopamine susceptible to thedisease. He started with a naturally derivedpesticide called rotenone, which was knownto cause mitochondrial damage and remainsone <strong>of</strong> several environmental factors thoughtto cause Parkinson’s.Sure, other researchers had met with somesuccess; but their techniques were limited.First <strong>of</strong>f, the dominant approach at the time—injecting rotenone directly into a mouse’sMitochondria, the green stuff, fusing (left) and undergoing fission (right). Fusing mitochondria are longer;fissioning ones are shorter; and the <strong>entire</strong> process is vital for cellular health.brain—indeed killed dopaminergic neurons,but it also obliterated plenty <strong>of</strong> other braincells not implicated in Parkinson’s. You mightexpect that a random and nonspecific braincell death would result no matter what toxinwas injected directly into the brain, especiallyin such high doses. Further, because <strong>of</strong>the injection method, such models damagedmitochondria only in the brain. Greenamyreand his colleagues believed, and emergingliterature suggested, that Parkinson’s patientshad systemic mitochondrial damage, not justdamage to the mitochondria in their dopaminergicneurons.Greenamyre and his Emory group examinedcells from humans—Parkinson’s patients.They determined the degree to which mitochondrialfunction was inhibited and thenslowly but surely found the right level <strong>of</strong>rotenone, administered intravenously, to replicatein mice what they had found in people.Success here produced systemic mitochondrialdysfunction, hit only dopaminergic neurons,and produced Parkinsonian symptoms in themice. Even more useful: The mice developedtangles <strong>of</strong> alpha-synuclein proteins (the sameproteins that aggregate in the brains <strong>of</strong> humanParkinson’s patients) called Lewy bodies. Theresulting paper, published in 2000, remains themost cited in Nature Neuroscience.“This model was the first pro<strong>of</strong> <strong>of</strong> conceptthat generalized mitochondrial dysfunctioncould lead to very specific neural degeneration,”Greenamyre says. “And since thenthe genetics <strong>of</strong> Parkinson’s disease have reallyadvanced. Now we’ve found several genes that,when mutated, affect the mitochondria andproduce Parkinson’s.”The common thread, then, is that regardless<strong>of</strong> whether a particular case <strong>of</strong> Parkinson’sCENTER FOR BIOLOGIC IMAGING, UNIVERSITY OF PITTSBURGHis spurred by environmental toxins suchas rotenone or genetic mutations accumulatedover time, mitochondria remaincentral.“That’s true in many, probably themajority, <strong>of</strong> Parkinson’s cases,” Greenamyresays. “If you’re thinking about the pathogenesis<strong>of</strong> Parkinson’s, you can start thedegenerative process in numerous places.In some cases, it starts with the mitochondria,in some cases not. But mitochondriawill play a vital role in the disease at somepoint.”Charleen Chu is confi dent thatGreenamyre is right. So much so thatthe MD/PhD pr<strong>of</strong>essor <strong>of</strong> pathology andPIND me<strong>mb</strong>er is looking at five models<strong>of</strong> Parkinson’s, all <strong>of</strong> which affect themitochondriac’s favored organelle. To Chu,regardless <strong>of</strong> whether the models involve neurotoxinsor mutated genes, the upshot is thatsomething is quite wrong with mitophagy, theprocess by which damaged mitochondria aresafely destroyed. Or, if it’s not mitophagy perse that’s gone awry, it’s the related and equallyimportant cellular step <strong>of</strong> generating newmitochondria to replace the bad ones that havebeen eaten up.“We’ve studied the recycling <strong>of</strong> mitochondriafor some time,” Chu says. “And the questionwe’re faced with is, ‘Well, if mitophagy isgood for you, why, in some cases, does it causeneuronal cell death?’”In the spring, Chu and colleagues publisheda paper in Cell Death & Disease that gives someinkling <strong>of</strong> an answer. She found that there are24 PITTMED
which mitos meet each other, swap geneticmaterial, and separate could have a significantrole—when it goes wrong, that is—in theprogression <strong>of</strong> Parkinson’s.Sarah Berman (PhD ’98/MD ’00), a PINDme<strong>mb</strong>er and assistant pr<strong>of</strong>essor <strong>of</strong> neurology,became interested in mito dynamics andParkinson’s as a grad student here at <strong>Pitt</strong>.During her training, she studied reactive oxygenspecies (ROS), the nasty stuff that mitochondriamake when the energy productionprocess has gone bad. The idea was that ROSwas one <strong>of</strong> the factors that accounted for theslaughter <strong>of</strong> dopamine neurons in Parkinson’s.By Berman’s grad student days, this wasimportant and interesting, but no longer agroundbreaking concept. The body <strong>of</strong> evidencewas strong suggesting that trouble withcomplex I and, subsequently, mitochondrialrespiration, was a precursor to the development<strong>of</strong> Parkinson’s. Berman, in her own lab,decided to delve a bit more deeply into a differentaspect <strong>of</strong> mitos and Parkinson’s.“What I study is not so much respiration,but mitochondrial dynamics,” she says. “Itfind a way to alter the fission and fusion processes,to keep them healthy, “maybe then wecan develop a corrective therapy we can usebefore cells die and Parkinson’s develops.”Sitting in her <strong>of</strong>fice, Berman brings up amovie showing fission and fusion in real time.Mitos blink red and are tagged with a photoactivegreen fluorescent protein. When a pairfuses, yellow flashes on the screen.“See!” she says excitedly.“When the me<strong>mb</strong>ranes merge and the contentsget mixed up, the whole thing turns yellow.Then you see it divide into two, and that’sfission. You can monitor and quantify theseevents, and it’s neat. I mean, the geeky scientistthat I am, I look at this and think, It’s so cool! ”In the lab, Berman manipulates the proteinsresponsible for fission and fusion. Something abit unexpected happened after Berman startedplaying with those proteins—something thatcould, down the line, prove to be an importantstep in preventing cell death in Parkinson’sbefore the process really gets going.“When we add a fission inhibitor to therotenone model—and I wasn’t sure whichWhether a particular case <strong>of</strong> Parkinson’s is caused by toxins or genetics, mitochondria remain central.times when mitophagy outpaces a neuronalcell’s ability to regenerate mitochondria. “Inany recycling process, it’s not good enough todegrade the damaged stuff,” she says. “You haveto rebuild.” Neural cells require and burn moreenergy than other types <strong>of</strong> cells, making themparticularly dependent on mitos. When therearen’t enough mitos, there’s not enough energy;calcium is mishandled; death comes calling andcauses, in this case, neurodegenerative diseaseslike Parkinson’s.The discovery was based on a slightly counterintuitiveobservation: Inhibiting ERK, a proteinvital to cellular proliferation, resulted inrecovery <strong>of</strong> healthy, functional mitochondria.You see, neurons don’t proliferate, and slowingdown mitophagy gave them time for mitochondrialrepair.“When we inhibit ERK, not only do we slowdown degradation, we boost biosynthesis, andthe cells are completely protected,” she says.“In our other studies—we’ve used three geneticmodels and two toxin models—although mitochondriaare all impacted differently, all theseprocesses feed into this cycle <strong>of</strong> damage-mitophagy-resynthesis.”One path to a possible treatment forParkinson’s and like diseases is obvious: maintainthe balance between the destruction <strong>of</strong>ailing mitos and the generation <strong>of</strong> healthyreplacements. But doing so isn’t easy. BennettVan Houten, a <strong>Pitt</strong> cancer researcher featuredin the first installment <strong>of</strong> the Mitochronicles,calls mitochondrial degradation over time the“rust <strong>of</strong> life.” As time passes, he suggests, mitosmight just accumulate too much rust; and thebalance between death and resurrection, theintracellular circle <strong>of</strong> life, then becomes intractablyscrewed up.Chu puts it this way: “Even with increaseddamage, as long as the cycle is able to roll, youmight be able to compensate for it, and maybethat’s why diseases like these don’t present untildecades have passed.”Rolling. An interesting word choice in therealm <strong>of</strong> mitochondria. The conventional wisdom,for quite a while, was that these organelleswere static. They just hung about in the cell’sinterior and produced ATP. That view turnedout to be wrong. Mitos are not only very active;but movement, it is now known, is vital formitochondrial health. Further, the process bysounds like an obscure sort <strong>of</strong> thing, but thefact that mitochondria divide and fuse togetherand get transported up and down neurons, thisis really critical—in neurons, in particular.”Neurons, Berman says, are different fromother cells in that they require more energyto work, thereby increasing the importance<strong>of</strong> mitochondrial health. (Not that it is unimportantin other cells.) Further, she says, theprocess <strong>of</strong> fission and fusion, the movement<strong>of</strong> mitochondria from the cell body all theway down the axons to the synapses, seems tobe absolutely crucial to the life <strong>of</strong> the neuron.“If you take away the ability for themto divide, they don’t get down to the synapses,and the synapses don’t work right,” she says.“That’s really intriguing, because one <strong>of</strong> thethings that happens in Parkinson’s is that it’snot the cell bodies that die first, but the longaxon terminals. Something is happening veryfar away from the cell body, and then the celldies.”Berman uses an in vitro version <strong>of</strong>Greenamyre’s rotenone model to reveal themusic that makes mitochondria dance up anddown axons.Her hunch and hope are that, if she canway this was going to go—it turned out tobe protective. And if we prevented fission, wedidn’t lose the [axons]. Maybe we can alter theneuropathology, but we don’t know why thisis protective.“Yes, this is exciting,” Berman continues.“But at this point we have many more questionsthan answers.”In the world <strong>of</strong> mitochondria research—still a pretty young fi eld—questionsabound. Sruti Shiva has taken questionsabout the organelle’s role—with theaid <strong>of</strong> nitrite—to heart. As in heart attacks.Shiva is investigating how mitos, exposed toextra nitrite, protect cells from damage whenblood, and the oxygen it carries, floods backinto organs after a period <strong>of</strong> deprivation.For the past decade or so, it’s been knownthat nitrite, which is native to our bodies andalso found in green, leafy vegetables, plays arole in cell signaling. (It had previously beenconsidered nothing more than a physiologicallyinert product <strong>of</strong> nitric oxide.) What’s new,out <strong>of</strong> Shiva’s lab, is confirmation that increasingthe amount <strong>of</strong> nitrite in the bloodstreamcan increase the nu<strong>mb</strong>er <strong>of</strong> mitochondria in aFALL 2012 25
cell. (Shiva’s “cell <strong>of</strong> choice” comes from theheart.) And a boost in the nu<strong>mb</strong>er <strong>of</strong> mitoshas been shown to facilitate adaptation to highaltitude, increase energy efficiency, lengthenlifespan, and ease weight loss.An emerging bit <strong>of</strong> Shiva’s research (whichthe PhD assistant pr<strong>of</strong>essor <strong>of</strong> pharmacologyand chemical biology does as a me<strong>mb</strong>er <strong>of</strong> theVascular <strong>Med</strong>icine Institute), though, skipsover the nu<strong>mb</strong>er <strong>of</strong> mitochondria generatedby the introduction <strong>of</strong> extra nitrite. Rather,she has examined how nitrite spurs mito activity,leading to a series <strong>of</strong> cellular events that<strong>of</strong>fer protection to cells stressed by a lack <strong>of</strong>oxygen and its subsequent reintroduction inthe wake <strong>of</strong> a heart attack.“What we’ve found is that, if you give asmall dose <strong>of</strong> nitrite (pre-heart attack), you geta small burst <strong>of</strong> ROS [those unpleasant reactiveoxygen species] from the mitochondria,”she says. “This actually turns on a signalingcascade that signals for more protection. Itsomehow causes a cell to increase its production<strong>of</strong> antioxidants (counteracting thedamaging reactive oxygen species released bya stressor) and bump up protective signalingmessages.”Animal studies, Shiva says, have shown amassive reduction in organ damage.Pursuing and understanding the mechanismsby which nitrite and mitos conveycytoprotection are, naturally, preconditions tobringing any related therapy to the clinic. Thusfar, it appears that when nitrite is administeredbefore or during ischemia and reperfusion, itkeeps the now obviously vital mitochondrialcomplex I from going <strong>of</strong>f the rails. Keepingthat first step in ATP production on point,it seems, reduces the production <strong>of</strong> ROS andbolsters production <strong>of</strong> the antioxidants thattame those ROS that are released.The takeaway, Shiva says, is that a littlemore <strong>of</strong> something that’s already inside us canhelp our mitochondria protect our hearts froma second, and pr<strong>of</strong>oundly damaging, insult inthe wake <strong>of</strong> a heart attack.Children with mitochondrial encephalomyopathy(ME)—which causesseizures, headaches, strokes, lacticacidosis, dementia, and, typically, deathbefore age 20—are not born with evidence<strong>of</strong> the disease.“You’re born and deemed healthy at birthand then at some point later—in severe caseswe’re talking days or weeks after birth—the disease develops,” says Michael Palladino.“And being diagnosed with primary mitochondrialencephalomyopathy is pretty mucha death sentence, particularly in its [veryearly] childhood-onset form.” The <strong>Pitt</strong> associatepr<strong>of</strong>essor <strong>of</strong> pharmacology and chemicalbiology notes that when faced with ME,clinicians prescribe cocktails <strong>of</strong> vitamins andother supplements, but, “No one has any realconfidence that those are anything near a cureor even <strong>of</strong> any therapeutic value.” Yet the diseaseis horribly painful, he says, and “a childcould linger for a couple <strong>of</strong> months, a couple<strong>of</strong> years, maybe more.”Palladino has developed the only animalmodel <strong>of</strong> ME with a mitochondrial DNA(mtDNA) mutation; he uses Drosophila, thefruit fly. There are about 200 different suchmutations that cause ME, which affects one in11,000 children. Until Palladino’s flies camealong, it was difficult to study the disease.The mutation in Palladino’s flies causesthem to have poor motor control and exceedinglyshort lives. It was serendipity that ledPalladino to this particular strain. He andme<strong>mb</strong>ers <strong>of</strong> his lab were studying a fly model<strong>of</strong> neurodegeneration. (He is also a me<strong>mb</strong>er <strong>of</strong>PIND.) The process <strong>of</strong> sorting out its genetic“So we went from having very little interest in mitochondria to BOOM.”makeup led to the discovery that a mutation<strong>of</strong> the ATP6 gene in the fly’s mitochondriawas also causing symptoms akin to the rare butdevastating MEs that affect humans.“And this put us in a beautiful place,”Palladino says. “There’s all this interest inhuman primary mitochondrial encephalomyopathies,but no one had a model system<strong>of</strong> them. So we went from having very littleinterest in mitochondria to BOOM. We had tobecome experts in this pretty quickly, becausewe had a novel resource that no one else in theworld had.”The most important feature <strong>of</strong> the model,says Palladino, is the fact that endogenousmitochondrial diseases are progressive. Yes,the basics can be studied in vitro, but to getthe full picture <strong>of</strong> how such diseases take rootand devastate over time, you need to look at aliving creature.So with a compelling and unique model,Palladino has set <strong>of</strong>f to find some sort <strong>of</strong>viable treatment for the disease. And he’smade some progress.The first front is pharmacological therapy.Drug screening has yielded a handful <strong>of</strong> compoundsthat somewhat improve the condition<strong>of</strong> Palladino’s sick flies.“The drugs we’ve identified do make theanimals better—they live 5 to 8 percentlonger, and we’ve seen a 10 to 15 percentimprovement in locomotor function,” he says.“But the thing about pharmacological therapiesis that these are such complex diseases thatfew people think there’s going to be a magicpill that fixes them. The rationale is that mitochondriado so many important things thatthere can’t be just one target. A large cocktail <strong>of</strong>drugs probably wouldn’t even work.”Knowing this led Palladino to aim closer tothe source: mtDNA. Scientists have becomeadept at inserting and removing components<strong>of</strong> nuclear DNA, but they’ve had lesssuccess directly affecting the mitochondrialgenome. So although he knew his target, hewas unsure about the best weapon to deploy.Palladino chose allotropic expression. Theidea here—and this is a technique developedabout 15 years ago at Colu<strong>mb</strong>ia <strong>University</strong>—is that while no one knows how to directlymanipulate mtDNA, it is possible to tweakthe nucleus. Before inserting a gene into thenucleus, a researcher can attach chemicaldirections that will drag the protein, generatedby the gene in the nucleus, intothe mitochondria.“We’ve made some hugeimprovements to the efficacy <strong>of</strong> this process,”Palladino says. “We’re getting 10 to 15 percentrescue in longevity and 35 to 45 percentimprovements with locomotor function.“Within a year or two, we’re going to havesome real successes here. Our mutant’s diseaseis so incredibly severe—worse than it everis in humans—and we’re very excited aboutthe levels <strong>of</strong> improvement we’ve seen so far.Nevertheless, we’re pushing for a 40 to 50percent improvement in longevity.“We’d like to achieve that before we publish,and I think we can.”Palladino is so excited about the therapy’spotential—an excitement that seems bothnatural and a possible consequence <strong>of</strong> theespresso machine in his <strong>of</strong>fice—that he’salready in the early stages <strong>of</strong> planning forclinical trials.Why so optimistic? “Why not?” Palladinoasks. “It keeps you motivated!” For further mitochondrial motivation,visit www.upci.upmc.edu/trmad/26 PITTMED
Nationally, the ecosystems thatsupport getting scientific discoveriesinto the clinic have beenpretty fragile. A few years ago,the NIH stepped in to help.CAROL HAVENS/GETTY IMAGESSURVIVAL OFTHE FUNDEDBACK FROM THE BRINKOF EXTINCTIONBY ELAINE VITONEIt begins with a good idea. With a little luck and a lot <strong>of</strong> sweat,this idea grows into a fully realized and hard-won laboratorydiscovery. Then, with more luck and, yes, more sweat,that good idea in basic science could develop to become a humantrial and gestate for a while, experimentally, in the clinic. And, withtime, effort, resources, and sweat, sweat, sweat, it could prove itsworth—improve patients’ lives—and gain acceptance in the medicalcommunity. Eventually, our brave young idea could mature into anadvancement that improves human health on a grand scale.This is translational science—a bit <strong>of</strong> a buzz term, really.Simply put, it’s the process by which researchers work to bringnew science to the clinic. One key group <strong>of</strong> researchers onwhom we’ve relied to do this—physician-scientists, the MDs whoFALL 2012 27
oth treat patients and study their ailments—are a vanishing breed. And with their staplefood—federal research funding—in such shortsupply, it’s no wonder.Not only that, but medical research hasbecome extremely complex. This has made itmore difficult to learn all the skills needed “onthe job,” as Elias Zerhouni, former NationalInstitutes <strong>of</strong> Health (NIH) director, notes in a2005 New England Journal <strong>of</strong> <strong>Med</strong>icine op-ed.Increasingly, translational and clinical researchrelies on technology and on specialization inmultiple areas. Thus, game-changing discoveriesin medicine <strong>of</strong>ten emerge from novel partnerships—peoplefrom disparate disciplinescoming together to dream up <strong>entire</strong>ly newapproaches to old problems.Of course, the business <strong>of</strong> getting hatchlingsout <strong>of</strong> the lab and into the clinic requiresmoney to support the pr<strong>of</strong>essionals who undertakeyears <strong>of</strong> study and training. It requiresmoney to staff and sustain labs. Money torecruit patient volunteers; collect samples fromthem; and crunch, troubleshoot, and interpretthe data. Biomedical and behavioral researcheats funding as insatiably as a round-the-clocknoshing newborn.But what’s even more costly? Scientific inertia.Good ideas stuck forever in the nest.The stakes are high. Zerhouni notes in his2005 op-ed that this country’s medical andpublic health practices “must undergo a pr<strong>of</strong>oundtransformation in the coming decadesif we are to succeed in providing access to carefor all Americans at reasonable costs.”In 2006, the NIH created a special set <strong>of</strong>funds to support the delicate ecosystem thatscientists who do this work need to thrive.Among their longest-running awardees is the<strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh–based Clinical andTranslational Science Institute (CTSI). <strong>Pitt</strong>and its local partners have received some $150million for its CTSI since 2006. Today, CTSIsupports hundreds <strong>of</strong> researchers by fundingpilot studies, core laboratories, statistical support,regulatory assistance, study-volunteerrecruitment, education for researchers at allstages <strong>of</strong> their careers, and more.In addition to doing its level best to incubatephysician-scientists and their partners,CTSI has helped make <strong>Pitt</strong> a hospitable environmentfor getting great ideas into the clinic.Robert Arnold, the Leo H. Criep Pr<strong>of</strong>essor<strong>of</strong> Patient Care and pr<strong>of</strong>essor <strong>of</strong> medicine, saysCTSI resources have been invaluable in supportinghis own translational science efforts.And, more importantly, the institute’s educationprograms (18) deserve credit for <strong>Pitt</strong>’ssuccess in attracting a nu<strong>mb</strong>er <strong>of</strong> stellar juniorfaculty and fellows to his department.“To go someplace that has a fabulous groupthat has organized mentoring and meetingsevery month, where people get to present theirresearch, where they have grant reviews andthey urge people to involve statisticians early intheir project? That’s a great way to make surethe project is structured so that it’s most usefuland most likely to get good results.“There are few places in the country thathave that. It’s so hard to get funded. These arethe things that help people succeed.”Throughout the last six years, CTSI hasprovided crucial support in such discoveries asthe possible gene-regulation role <strong>of</strong> usRNAs(small strands <strong>of</strong> RNA once dismissed asmolecular junk) and the part polyomavirusplays in Merkel cell carcinoma.A few years ago, CTSI helped a team <strong>of</strong>researchers try an experimental treatment forparalysis—a brain-computer-interface device,in its first-ever human test drivers (patientswith epilepsy who volunteered to have theirneurological signaling studied during hospitalstays for seizure-mapping). CTSI seed moneyand regulatory support helped the researchersland an NIH grant, and, long story short, theresearchers’ hard work culminated in Octoberin an exciting moment: A man with paralysismoved a robotic arm with his thoughts, reachingout to his girlfriend’s hand for the first timein seven years. (See our Investigations story inthe Spring 2012 <strong>issue</strong>.)And the good ideas keep germinating. Hereare just a few examples <strong>of</strong> translational scienceresearch coming <strong>of</strong> age here at <strong>Pitt</strong>.Finally AirborneTwenty years ago, Don DeFranco and SelmaWitchel (MD ’78, Fel ’83) began their translationalscience journey at, <strong>of</strong> all places, alittle league game in Churchill, Pa. They weresitting on the sideline—each had a son in thegame that they were pretending to watch.“We were taking turns to see who was atbat,” admits Witchel.“We were both reading articles,” saysDeFranco, “and I looked over, and her articlehad words that I recognized.”Ever since, DeFranco, a molecular endocrinologistand pr<strong>of</strong>essor and vice chair <strong>of</strong> educationin <strong>Pitt</strong>’s Department <strong>of</strong> Pharmacologyand Chemical Biology, and Witchel, associatepr<strong>of</strong>essor <strong>of</strong> pediatrics and director <strong>of</strong>the pediatric endocrinology fellowship programat Children’s Hospital <strong>of</strong> <strong>Pitt</strong>sburgh <strong>of</strong>UPMC, have wanted to collaborate on theircommon ground—glucocorticoids and steroidphysiology.Often, in response to sepsis and traumaticbrain injury, blood levels <strong>of</strong> free cortisol—asteroidal hormone that (among other functions)keeps inflammation in check—increase.For good reason: Inflammation can be a criticallyill patient’s undoing. But some patientshave much lower total cortisol levels thanothers. So, in the 1970s, researchers triedadministering drugs related to cortisol (syntheticglucocorticoids) to patients with lowerlevels <strong>of</strong> the hormone; the researchers foundthat some improved. But in the coming years,other studies showed that there were too manycomplications, including infections and highblood sugar. The practice has been debated inthe literature ever since, its favor swinging backand forth like a pendulum.Today, glucocorticoids are again used inthese critically ill patients, albeit in smallerdoses than they were 40 years ago. Yet thedebate has hardly been put to rest. “Somepatients it helps, some patients it doesn’t,” saysDeFranco. “There’s still no consensus abouthow to best evaluate for adrenal insufficiencyduring clinical illness.”DeFranco and Witchel thought: Ratherthan the amount <strong>of</strong> total cortisol in the bloodstream,wouldn’t it be more helpful to knowhow cortisol interacts with these patients’immune cells—how well it’s doing its job, atthe molecular level?Interdisciplinary collaborations are amongthe hardest to get <strong>of</strong>f the ground. To win afederal grant, you need pilot data to proveyour idea has wings, and that takes money.Ideally, you have a larger effort to draw on—abig, preexisting, funded study that dovetailsinto a little spin<strong>of</strong>f study. First-<strong>of</strong>-their-kind,fledgling ideas generally aren’t so lucky.Such was the case with DeFranco’s andWitchel’s brainstorming. Then they heardabout a CTSI pilot fund for investigationsexploring new territory and promoting newresearch partnerships. Right around the sametime, Cristina Candido-Vitto (Fel ’09), then afellow, expressed interest in doing the legworkfor such a project. The team applied for andreceived their award in 2008 and, after years <strong>of</strong>talking about it, finally got to work.The researchers studied white blood cellstaken from pediatric critical-care patients(using very small samples, collected whileDANIEL J COX/GETTY IMAGES28 PITTMED
What’s more costly than the business <strong>of</strong> moving breakthroughs from the labinto the clinic? Scientific inertia. Good ideas stuck forever in the nest.other blood draws were already being taken,so as not to interfere with care). They lookedat what was actually going on within the cell:Were glucocorticoid receptors in the rightplaces, in adequate nu<strong>mb</strong>ers? Was cortisolbinding to them in the cytoplasm, thenmoving to the nucleus—all-important stepsthat enable a molecule to give the cell itsmarching orders?They found that the glucocorticoid receptorprotein maintained some function—itwas indeed binding to the hormone andmoving from the cytoplasm to the nucleus.However, in these critically ill patients, thelevel <strong>of</strong> receptors and their ability to bind weredecreased in the early stages <strong>of</strong> illness.“It looks like these kids don’t havethe capacity to respond to glucocorticoidtherapy,” says DeFranco.“The physiologic signifi cance <strong>of</strong> thedecreased nu<strong>mb</strong>er <strong>of</strong> receptors is unclear. ...”says Witchel. “[Still] they’re missing part <strong>of</strong>the effector mechanism they need to get aresponse.”The team has written a review article on thetopic. They hope their findings might eventuallycontribute to a better blood test and takeFALL 2012 29
the guesswork out <strong>of</strong> who stands to benefitfrom anti-inflammatory hormones, what dosageis beneficial, and how long patients shouldcontinue the therapy.Before CTSI’s pilot-funding program andothers like it across the country, there wereno federal mechanisms to bring good ideaslike DeFranco and Witchel’s to fruition. Apartfrom a few very targeted grants, there was virtuallyno way to fund a mini-pro<strong>of</strong>-<strong>of</strong>-conceptproject to demonstrate whether a study wasworthy <strong>of</strong> funding. It was classic chicken andegg.“Without CTSI,” says DeFranco, “thiswould have been just another dinner conversation.”Different FeathersSay you are unlucky enough to have a badfall, or a run-in with a piece <strong>of</strong> constructionequipment, or any <strong>of</strong> the other cringe-worthyscenarios that could eventually land you inrecovery from orthopaedic surgery, waking upwith what arguably amounts to the worst <strong>of</strong>the worst <strong>of</strong> post-op pain. The good news isthat you live in a time when there are drugsavailable to keep you comfortable. For mostpeople, opioids are very good at lulling a pr<strong>of</strong>oundlytraumatized body.But for some people, they’re so good thatthe body neglects one all-important function:breathing.At that point, an alarm sounds—a signalthat your blood-oxygen level is too low.The team <strong>of</strong> nurses caring for you leaps intoaction. They’ll lift your chin and draw yourjaw uncomfortably forward—to open yourairway and, more importantly, to annoy yourgroggy body into breathing again. If theyhave to, they’ll put an endotracheal tubedown your throat.If all else fails, you’ll receive what’s calledan opioid rescue. Naloxone, a commonoption, will nix that whole not-breathingthing for a full hour. Problem is, it will alsoget rid <strong>of</strong> what you, on that most unluckyday, want most: pain relief.Genetic and other factors that contribute tohow well opioids manage individuals’ pain havebeen widely studied—but not the risk factorsfor respiratory depression. Which means wehave absolutely no way <strong>of</strong> knowing who is infor a particularly rude awakening after surgery.“I did the math,” says Will Lariviere,assistant pr<strong>of</strong>essor <strong>of</strong> anesthesiology and neurobiologyin the School <strong>of</strong> <strong>Med</strong>icine, “and itturns out there’s a quarter-million Americansgetting a naloxone rescue for pr<strong>of</strong>ound respiratorydepression each year—and that’s basedon conservative estimates.”Lariviere is an expert in the responsesto pain and analgesia and the interactionsbetween stress and pain systems—in animalmodels. But how respiratory depression looksin the clinic never really came to life forhim until a few years ago, when a colleagueintroduced him to nurse anesthetist RichHenker, a pr<strong>of</strong>essor and international educationcoordinator for acute and tertiary care inthe School <strong>of</strong> Nursing. They got to discussingrecovery in the postoperative environment.“Every time we met,” says Lariviere, “Iwould realize, Oh my gosh, the nurses are runningaround like crazy trying to keep peoplebreathing.”“Will brings new perspective to my clinical[experience],” says Henker. “I don’t thinkabout it. I’m like, Well, no, that’s just whatwe do.”The problem with opioid-induced respiratorydepression has been cited numeroustimes in nursing literature. And now, thanksto a 2009 CTSI pilot grant, this unlikely pairing<strong>of</strong> a bench scientist and a nurse anesthetistis finally confronting a problem that peerreviewedliterature aimed at MDs has yet toaddress: A side effect <strong>of</strong> analgesia, respiratorydepression, is actually dictating the quality <strong>of</strong>care that some patients receive.Lariviere is hunting for relevant genetictargets in animals; Henker is testing forthose targets in humans—orthopaedic-surgerypatients with lower-li<strong>mb</strong> fractures, tobe precise. So far, they have identified severaldistinct genotypes that respond uniquely toopioids, either in terms <strong>of</strong> analgesia, respiratorydepression, or both. They have presentedtheir findings nationally and published twicein Biological Research for Nursing. Their workis ongoing while they apply for larger grantsfrom the NIH.The goal, says Henker, is to tailor analgesiafor optimal effects. Who needs moreopioid, who needs less? And whom can theyflag, based on genetic risk factors, as a patientwho would fare much better on a nonopioidmedication?“The goal is to try to get rid <strong>of</strong> pain forthese people.”Eagle EyeMelanoma is unique among cancers in thatit’s right there where you can see it—a newmark on your skin, a visual reminder thatyou’ve got to get it checked out. The rubis in the getting-it-checked-out part. Most<strong>of</strong>ten, you’ll see a primary care doc, whothen has to decide whether to call a dermatologist.Who will then decide whetheryou need a biopsy. Which will take time toprocess in pathology. And all the while, theclock is ticking.“Melanoma can grow from a curablelesion to an incurable one while you’re waitingfor an appointment,” says Laura Ferris,an MD assistant pr<strong>of</strong>essor <strong>of</strong> dermatology.False-alarm biopsies cause patients undueexpense, not to mention discomfort andworry, and that thought weighs heavily onthe mind <strong>of</strong> your front-line clinician. Butwhat if there were a virtual second opinionavailable, a tool to aid in the determination <strong>of</strong>whether to biopsy you right then and there?Ferris is working with MahadevSatyanarayanan, pr<strong>of</strong>essor <strong>of</strong> computer scienceat Carnegie Mellon <strong>University</strong>, ona new use for his Web-based application,dubbed Diamond. The application can takeany image you give it, compare it to othersin a database, and display the ones it mostclosely rese<strong>mb</strong>les. Diamond has already beendeployed in a nu<strong>mb</strong>er <strong>of</strong> ways, from clarifyingmammogram results to spotting terroristsin video-surveillance footage.Ferris has uploaded some 1,000 images<strong>of</strong> UPMC patients’ skin lesions withknown biopsy results (with patient identitiesremoved). Funding from CTSI’s NovelTechnologies Core—which supports newbiotech efforts—allowed the researchers tohire a programmer to harness this resourcefor melanoma screening. Once complete,this latest iteration <strong>of</strong> Diamond will be ableto find the image best rese<strong>mb</strong>ling a newlesion’s unique features—shape, color, pigmentpatterns, blood vessels, and so on. Inaddition to its great potential as a diagnosticaid for both primary docs and dermatologists,it’s a promising dermatological teachingtool.And yet it almost didn’t happen.Ferris and Satyanarayanan began theproject at a privately funded laboratory. Thatarrangement fell through when it becameclear that their mission—to make Diamondopen-sourced, for anyone to use and benefitfrom—didn’t jibe with that <strong>of</strong> their sponsor.CTSI’s Novel Core was uniquely suited tothe project, says Ferris. “There’s really not alot <strong>of</strong> funding to help you sit down and buildsomething like this from scratch.” CIDDE/JOE KAPELEWSKI30 PITTMED
Since its establishment six yearsago, CTSI has enabled investigatorsto conduct 5,200 studies.CTSI: A BIRD’S EYE VIEWThe <strong>University</strong>’s Clinical and Translational Science Institute(CTSI), a National Institutes <strong>of</strong> Health (NIH)–funded effort,helps get clever ideas <strong>of</strong>f the ground. With its support, <strong>Pitt</strong>has become an incubator <strong>of</strong> physician-scientists and othersengaged in bringing promising discoveries to the clinic, wherethey can help patients. Here are a few ways CTSI is feedingthe flock.• CTSI’s Institute for Clinical Research Education has growninto one <strong>of</strong> the premier clinical and translational researchtraining programs in the country, now <strong>of</strong>fering 18 academiccareer-development programs for grad students, med students,residents, fellows, postdocs, and faculty in all phases<strong>of</strong> their careers. For example, in 2011, CTSI’s PhD Program inClinical and Translational Science graduated its first threestudents. Another 11 are in the queue. (The Institute has evenengaged more than 9,000 high school students. CTSI runs amobile science lab—it’s a truckload <strong>of</strong> fun.)• In the past six years, CTSI programs enabled 2,100 investigatorsto conduct 5,200 studies.• Last fiscal year, CTSI-funded facilities provided quantitativeanalysis <strong>of</strong> more than 6,000 clinical samples, bioinformaticsanalysis for 43 studies, and assistance with regulatory compliancefor 69 investigators.• Each year, CTSI fills more than 150 requests for assistancewith study design, biostatistics, and epidemiology support.• ProDy, a free and open-source molecular-systems-modelings<strong>of</strong>tware package (developed by <strong>Pitt</strong>’s Ivet Bahar with CTSIsupport), has been downloaded more than 10,000 times in thepast two years.• At last count, there were more than 34,000 people enrolled inthe CTSI Research Participant Registry. —EVFALL 2012 31
ATTENDINGRuminations on the medical lifeFranklyn Boothe is one <strong>of</strong> hundreds <strong>of</strong> young people who spentthe summer working with School <strong>of</strong> <strong>Med</strong>icine researchers.SUMMER TO REMEMBERCURIOSITY PIQUED AT PITTBY JENELLE PIFERPHOTOGRAPHY BY MARTHA RIALIf during high school or early in your undergraduate years you decideyou want to become a doctor, the first thing an advisor will likelytell you is: You’re going to need to do a lot <strong>of</strong> research. That’s what happenedto Franklyn Boothe, an undergraduate and senior at <strong>Pitt</strong>. Research, hethought back in 10th grade, it sounds interesting. But what is it?“It’s this mysterious thing you’re supposed to do,” says Boothe, “but theydon’t tell you exactly what it entails.”Having spent this summer (his second in a row) studying how antipsychoticdrugs work in schizophrenia, Boothe knows this: “You just have tostart small and ask a lot <strong>of</strong> questions.”32 PITTMED
He got his start in the lab with David Lewis,an MD, director <strong>of</strong> <strong>Pitt</strong>’s National Institute <strong>of</strong>Mental Health–funded Conte Center for theNeuroscience <strong>of</strong> Mental Disorders, chair <strong>of</strong> <strong>Pitt</strong>’sDepartment <strong>of</strong> Psychiatry, and UPMC EndowedPr<strong>of</strong>essor <strong>of</strong> Translational Neuroscience. Bootheis one <strong>of</strong> 15 undergraduates participating in theConte Center’s summer research fellowship.Lewis says he hopes the program will engagestudents both “intellectually and affectively”by showing them the path discoveries take tobecome treatments, as well as “the importance<strong>of</strong> developing new treatments from a humanperspective.” Fellows attend weekly lectures onfaculty research, as well as clinical sessions wherethey interact with patients face to face.The latter was eye-opening for Boothe. Hemet high-functioning schizophrenia patientsand patients so overwhelmed by delusions theycouldn’t maintain relationships or hold downjobs. “It really puts things in perspective andgives a face to what you’re working for,” he says.“It’s easy to lose track <strong>of</strong> why we’re sitting in alab and pipetting for several hours. It’s to tryand improve people’s lives.”Boothe’s first days in the lab were among histoughest. “It’s kind <strong>of</strong> like being thrown into aquantum physics class when you’ve never takenphysics before,” he says. He had to acquire thelingo, memorize the protocol, and master newtechniques.This summer, however, he’s working almostindependently. He’s designed an experimentto help determine how mitochondria changein schizophrenia. The Lewis lab had alreadydefined an abnormality that may cause lessenergy to be produced, fewer neurons to fire,and problems to occur in systems like workingmemory. Still, Boothe says, there couldbe confounding factors. Not enough researchhas been done into how antipsychotic drugsaffect mitochondria, and researchers can’t say,with certainty, that it’s the disease causing thechange, not the drug. Boothe’s preliminarydata indicate the drug is not responsible.It’s the first major study he’s conductedfrom start to finish. “It’s been really gratifyingto not only be able to do this on my own butto get good results,” Boothe says.The big picture, Lewis says, is that mentalillnesses and addictive disorders account for40 percent <strong>of</strong> all the years lost to prematuredeath from disability in the United States.“The problem is big,” he says, “and we needthe best and the brightest now and in thefuture to consider it.”For a career path as demanding asresearch, students have to take someinitiative, says <strong>Pitt</strong>’s Guy Salama, aPhD. In other words, they need to knock onsome doors.And the busy scientists on the other side?They need to be willing to answer.“People who have decided to dedicate theirlives to research are few and far between, soyou have to cater to them a bit and encouragethem. Otherwise, it’s not going to happen,”says Salama, who is a pr<strong>of</strong>essor <strong>of</strong> cell biologyand mentor in the <strong>Pitt</strong>sburgh Research andInvestigation Summer Experience (PRISE)program, a summer cardiology fellowship forundergrads.Zane Kalik, a rising senior at YoungstownState <strong>University</strong> (YSU) and current PRISEfellow, came knocking early on. “It doesn’tmatter where you start, just get a good researchfoundation as early as possible,” says Kalik,who started in an environmental biology labas a freshman.When he was a junior at YSU he caughtSalama’s attention. Kalik’s mentor Carl Sims,a former <strong>Pitt</strong> postdoc with Salama who laterjoined the faculty at YSU, had recently died.Kalik had been so well trained he was able topick up where Sims left <strong>of</strong>f. Kalik was usingequipment that allowed him to study electriccurrents in the heart, and how they sync tocreate irregular heartbeats called arrhythmias.Salama was impressed and invited the buddingresearcher to speak at <strong>Pitt</strong>’s CardiovascularInstitute.When Kalik heard about the PRISE fellowship—fundedin part by the American HeartAssociation—he applied, was accepted, andspent the summer at <strong>Pitt</strong> researching a newdrug to prevent atrial fibrosis.“The cardiac action potential is a symphony<strong>of</strong> ion currents,” says Kalik, now in hissecond year with PRISE and one <strong>of</strong> six fellows.“All those currents work together to generateour heartbeat, and it’s a very beautiful process.”But this electric chorus can get thrown out <strong>of</strong>whack when deposits <strong>of</strong> collagen develop inthe atria because <strong>of</strong> injury or aging. (This iscalled fibrosis.) The heart can then becomehighly susceptible to atrial fibrillation, themost common arrhythmia. A hormone calledrelaxin, however, seems to prevent this; Kalik istrying to figure out how.“Zane is really unusual. He’s just totallydevoted. He spends an enormous [nu<strong>mb</strong>er]<strong>of</strong> hours to try to crack a difficult nut,” saysSalama. Kalik’s typically in the lab between 12and 15 hours per day, a habit he attributes tohis former mentor Sims. Kalik says the morecells he can study, the better his data.“A summer—a lot <strong>of</strong> people say—it’s notenough time. I think it is,” he says. “You justhave to use the time, and you have to reallywork at it.”The Chang-Moore lab at <strong>Pitt</strong>—a tumorvirology lab run by National Academy<strong>of</strong> Sciences me<strong>mb</strong>ers Patrick Mooreand Yuan Chang—took in a high schoolstudent this summer. It was the first time ithad done so. The researchers had decided toparticipate in the Doris Duke FoundationBirdy AssefaFALL 2012 33
Zane KalikAcademy for Clinical Research, an eight-weekprogram for young people from underrepresentedand disadvantaged groups. Programdirector Michael Lotze, <strong>Pitt</strong> pr<strong>of</strong>essor <strong>of</strong> surgeryand assistant vice chancellor, health sciences,reme<strong>mb</strong>ers when he first sidled up tothe research pair with the idea: “They said,‘Make sure you give us a good one.’”Birdy Assefa, an enthusiastic 16-year-old,did not disappoint.She found out about the program throughthe Jack Kent Cooke Foundation, which connectshighly motivated students with newopportunities as early as the seventh grade.Assefa met with her advisor there. “I told her,‘I’m interested in research, and I want to knowwhat that is.’” Her advisor directed her to <strong>Pitt</strong>.This summer Assefa immersed herself.Working alongside Chang at the bench everyday, Assefa studied a virus called the Merkelcell polyomavirus (MCV). The Chang-Moorelab had recently discovered that MCV causesan aggressive type <strong>of</strong> skin cancer called Merkelcell carcinoma, but the team is still working tounwrap the biochemical pathways responsible.“It’s really interesting, and there’s so muchto be done [in cancer research],” saysAssefa, who attended lectures and workedalongside 37 other scholars in this programand the <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburghCancer Institute Academy. Assefa, who’soriginally from Ethiopia, came to Americaat age 10 and now attends high school inArlington, Va.“She had the personality to be inquisitive,”says Chang, “She just came in withthat and leaves the lab with that.” Changreme<strong>mb</strong>ers one early interaction whenAssefa was learning to manipulate DNA:“She said, You know what they show inCSI [the TV show]? Does it really happenthat way? ”What’s great about the Doris Dukeprogram, Chang says, is it harnesses students’enthusiasm and teaches them whatscience is really about.Assefa now speaks fluently about the projectand her plan to go to med school one day.Lotze spearheaded the Doris Duke programat <strong>Pitt</strong> many years after spending two pivotalsummers in high school programs himself:“One at Northwestern <strong>University</strong>, where Iended up going to college and medical school,”he says, “and the other at Strong MemorialHospital in Rochester, N.Y. My first summerafter high school I worked in an operatingroom, and I ended up becoming a surgeon.”Beginnings, he says, are really important. NOT SO LAZY DAYSThe School <strong>of</strong> <strong>Med</strong>icine and partners <strong>of</strong>fer summer researchand enrichment opportunities for students 16 and older.UNDERGRADUATE• Center for Neuroscience at the <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburghSummer Undergraduate Research Program• Conte Center for the Neuroscience <strong>of</strong> Mental DisordersUndergraduate Research Fellowship• Models <strong>of</strong> Infectious Disease Agent Study (MIDAS) SummerResearch Program, MIDAS National Center <strong>of</strong> Excellence• <strong>Pitt</strong>sburgh Center for Kidney Research• <strong>Pitt</strong>sburgh Research and Investigation Summer Experience(PRISE) for Undergraduate Students, Cardiovascular Institute• <strong>Pitt</strong>sburgh T<strong>issue</strong> Engineering Institute Summer Internship Program• <strong>Pitt</strong>sburgh Tuskegee Prostate Training Program,Department <strong>of</strong> Pathology• Summer Premedical Academic Enrichment Program,Student Affairs/Diversity Programs• Summer Undergraduate Research Program,Departments and Divisions <strong>of</strong> Cell Biology and MolecularPhysiology, Cellular and Molecular Pathology, Immunology,Molecular Genetics and Developmental Biology, MolecularVirology and Microbiology, Molecular Pharmacology, andMolecular Biophysics and Structural Biology• TECBio, Department <strong>of</strong> Computational and Systems BiologyHIGH SCHOOL• Doris Duke Foundation Academy for Clinical Research• <strong>Med</strong>ical Explorers, Student Affairs/Diversity Programs• <strong>Pitt</strong>sburgh T<strong>issue</strong> Engineering InstituteHigh School Summer Research Internships• Summer Premedical Academic Enrichment Program,Student Affairs/Diversity Programs• <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh Cancer Institute Academy,Departments <strong>of</strong> Biomedical Informatics andComputational and Systems Biology, Hillman Cancer Center,Women’s Cancer Research Center• <strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh Health Career Scholars AcademyFor more information: www.howscienceworks.pitt.edu or Office <strong>of</strong>Science Education Outreach, M525A Sciafe Hall, 412-648-9572.34 PITTMED
98.6 DEGREESPeople and programsthat keep the schoolhealthy and vibrantBRIAN MATHIE UPMCLEMIEUX CENTERNEARS COMPLETIONBLOOD CANCER TREATMENTAND RESEARCH, IN ONE PLACEBY JENELLE PIFERIt was in the middle <strong>of</strong> his greatest seasonwith the <strong>Pitt</strong>sburgh Penguins that MarioLemieux was diagnosed with Hodgkin’slymphoma. That same year the hockey star(now cancer free) created the Mario LemieuxFoundation, which raises money for cancerresearch in Western Pennsylvania. In 2010,the foundation presented the <strong>University</strong> <strong>of</strong><strong>Pitt</strong>sburgh Cancer Institute (UPCI) andUPMC CancerCenter with $3 million, closinga fundraising campaign to build a newtreatment facility. UPMC then stepped inwith an additional $10 million investmentfor the Mario Lemieux Center for BloodCancers, now nearing completion.“We are thrilled that [Lemieux’s] name,which we associate with success, is going tobe associated with our center,” says NancyDavidson, the MD director <strong>of</strong> UPCI and <strong>Pitt</strong>pr<strong>of</strong>essor <strong>of</strong> medicine.The center, set to open this Dece<strong>mb</strong>er,will occupy the fourth floor <strong>of</strong> the HillmanCancer Center and serve an estimated25,000 patients a year. Employing a mixedusedesign, it will <strong>of</strong>fer comprehensive diagnosticand treatment services and access toclinical trials.“The center will allow us to bring together,in one place, the practitioners,researchers, andsupport services that arewrapped around the care<strong>of</strong> individuals with blood cancers in ourregion. It’ll help us to really optimize care,”Davidson says.From a patient’s perspective, this meansbeing able to enter the center and receivecomplete treatment services in one place.Usually, Davidson says, “you have to go tothis <strong>of</strong>fice to see the doctor, and you haveto go somewhere else for your blood drawing,and a third place to check out.” WhatUPCI wants is that when patients check in,they’re sent to the right place, and everything—physicians,therapists, staff to drawblood—comes to them.This patient-centered focus also extendsto the site’s operating hours, says StanleyMarks (MD ’73, Res ’76), director <strong>of</strong> clinicalservices, chief medical <strong>of</strong>ficer for UPMCCancerCenter, and clinical pr<strong>of</strong>essor <strong>of</strong> medicineat <strong>Pitt</strong>. “Many <strong>of</strong> the patients who havethese cancers, especially those who haveleukemia or those undergoing bone marrowtransplant, they’re in a situation where theyrequire care almost every day for long periods<strong>of</strong> time.”Typically these patients are treated in theinpatient setting, he adds. But the LemieuxCenter is joining a nationwide push totry to bring as much medicine as possibleinto the outpatient setting, which helpsoutcomes, minimizes hospital complications,and improves quality <strong>of</strong> life for manypatients. To do so, the center will be open 12hours each day, with weekend hours, too, soservices are continuously available to thosewho need them.The design also means more synergy forThe new Mario Lemieux Center for BloodCancers will be on the fourth floor <strong>of</strong> theHillman Cancer Center.new research: Researchers’ <strong>of</strong>fices are intermingledwith treatment rooms. “We alwayswant to be doing this in oncology. We are notsatisfied with where we are in the treatment<strong>of</strong> almost any kind <strong>of</strong> malignancy, and bloodcancers are no exception,” says Davidson.Mario Lemieux, chair <strong>of</strong> the MarioLemieux Foundation, says, “The translationalresearch component <strong>of</strong> our new bloodcancer center will help us take one stepfurther toward reaching our goal <strong>of</strong> findingcures for cancer.”BOOSTER SHOTIt’s not the most common husband/wife origin story, but Noel Gilletteand Lynn Daugherty Gillette (bothMD ’60) met in med school over a sharedcadaver in anatomy class.Noel Gillette, who practiced inMonroeville and Wilkinsburg, died inMarch. He considered himself a lifelonglearner and something <strong>of</strong> a teacher, as well.His donation to the School <strong>of</strong> <strong>Med</strong>icine,his own body, will allow him to carry onhis teaching role, says the Gillettes’ daughter,Susan Gillette Meer.“This [donation] allowed him to servehis dear alma mater. And, I think, a smallromantic side <strong>of</strong> it is knowing the possibilitythat friendship, or possibly love, couldbe found among the medical students inthe same way he and my mother foundtheirs,” she says. —Joe MikschFor more information on making a similardonation, go to www.ooas.pitt.edu/content.asp?id=1735MONTH FALL 2005 2012 35 1
ALUMNI NEWSCLASS NOTES’70s Ed<strong>of</strong> medicine at the <strong>University</strong> <strong>of</strong> Washington who hasBoyko (MD ’79), a pr<strong>of</strong>essordevoted two decades to epidemiologic research, is theVA investigator for the Millennium Cohort Study, thelargest prospective health project in military history.Using volunteers’ self-reporting as well as Department<strong>of</strong> Defense medical records, Boyko and his team areevaluating the long-term physical and mental healtheffects <strong>of</strong> military service, looking for links betweenmilitary experience and physical and mental health outcomesin their 150,000 study participants. “The mainexposure everyone’s concerned about is deploymentto co<strong>mb</strong>at zones,” he says. So far, they’ve found thatin this population (active duty military and reservists,most in their 20s and 30s), PTSD has a strong link totype 2 diabetes—even stronger than the previouslyestablished link between depression and type 2 diabetesin the general population.Boyko is also a principal site investigator forthe Million Veteran Program, a VA patient biorepositoryin the making, which now has more than 70,000enrollees toward its million-enrollee goal. (<strong>Pitt</strong> is one<strong>of</strong> the study’s 40 sites, he notes.) Once complete, thebiorepository will be a treasure trove for epidemiologicresearchers, with blood samples, questionnaires onhealth and lifestyles, and health care records. “Typically,you need to get permission from regulatory bodies; youneed to go out and recruit subjects; and you need to setup your processing and analysis <strong>of</strong> data. All this couldtake five years. A biorepository should create considerablesavings in time,” he says.’80s Imagine you could build a neworgan from scratch. David Gerber (MD ’89, ClinicalTransplant Fellow ’98), pr<strong>of</strong>essor <strong>of</strong> surgery, surgicaldirector <strong>of</strong> liver transplantation, and chief <strong>of</strong> abdominaltransplant surgery at the <strong>University</strong> <strong>of</strong> North CarolinaSchool <strong>of</strong> <strong>Med</strong>icine, hopes to make that dream a reality.Gerber is researching stem cells and nanotubes to meetthe needs <strong>of</strong> many patients suffering from organ failure.“Our current strategies are not meeting this clinicalgap,” he says. T<strong>issue</strong> engineering has a nu<strong>mb</strong>er <strong>of</strong>potential applications, including the creation <strong>of</strong> artificialpancreases to secrete insulin in diabetics or designingvascular grafts to repair blood vessels. Gerber’s work inFAMILIAL TALESthis field is focused on the three-dimensionalgrowth <strong>of</strong> neo-t<strong>issue</strong>s—he’s currently principalinvestigator for more than 15 clinical trialsaimed at better outcomes for kidney- and livertransplantpatients. “The longer-term goal isthe development <strong>of</strong> implantable biologic units,”he says.Small’90s Language is among themany things that make us distinctly human. Studying how ourlinguistic faculties develop is one <strong>of</strong> the primary concerns <strong>of</strong>Steven Small (Neurology Resident ’91), editor in chief <strong>of</strong> Brainand Language and founder <strong>of</strong> the Society for the Neurobiology<strong>of</strong> Language. After his residency, Small took on a nu<strong>mb</strong>er <strong>of</strong>positions in <strong>Pitt</strong>’s Departments <strong>of</strong> Neurology, Psychology,Communication Sciences and Disorders, and IntelligentSystems, and also was a founding me<strong>mb</strong>er <strong>of</strong> the <strong>Pitt</strong>-CarnegieMellon <strong>University</strong> Center for the Neural Basis <strong>of</strong> Cognition.Now, as pr<strong>of</strong>essor and chair <strong>of</strong> neurology at <strong>University</strong> <strong>of</strong>California, Irvine, Small studies language and hand-motorrecovery following stroke. By observing the natural patterns <strong>of</strong>recovery, Small has devised various interventional techniquesthat could speed up the healing process.A decade ago, only about 10 percent <strong>of</strong> plastic surgeonswere women. But that’s changing rapidly, says Anureet Bajaj(MD ’96). Today, 30 percent <strong>of</strong> all plastic surgery residents arewomen. Bajaj, assistant medical editor <strong>of</strong> Plastic Surgery Newsand chair <strong>of</strong> the Women Plastic Surgeons Steering Committeefor the American Society <strong>of</strong> Plastic Surgeons, is doing her levelbest to fuel the fire. The Oklahoma City–based reconstructiveand cosmetic plastic surgeon organizes teleconferences eachmonth for the committee, as well as workshop luncheons atthe society’s annual meeting, covering such topics as timemanagement, legal <strong>issue</strong>s, work/life balance, and socialnetworks and practice. She’s now organizing the committee’s2013 retreat, slated for sometime this spring in Las Vegas, Nev.Sandeep Nathan (Internal <strong>Med</strong>icine Resident ’98), directorAs kids, neither Susan Dunmire (MD ’85) nor Samuel A. Tisherman (MD ’85,Res ’93, Fel ’94) got to watch much <strong>of</strong> the Super Friends or other Saturday morningcartoons. They were <strong>of</strong>f with their dads, Lester A. Dunmire (MD ’48) andSamuel E. Tisherman (MD ’55), on rounds.Little Susan and Sam grew up and met decades later at the welcoming reception<strong>of</strong> <strong>Pitt</strong> med’s Class <strong>of</strong> ’85. They got to know each other. They fell in love. They chosespecialties. (Susan is a <strong>Pitt</strong> associate pr<strong>of</strong>essor <strong>of</strong> emergency medicine; Sam a <strong>Pitt</strong>pr<strong>of</strong>essor <strong>of</strong> critical care medicine, as well as surgery.) Surgeon Lester Dunmire diedthis summer, but the familial medical tale continues as Susan and Sam’s oldest,Robert Tisherman (Class <strong>of</strong> 2017), begins his first year at <strong>Pitt</strong> med. Robert is the 15thme<strong>mb</strong>er <strong>of</strong> his family to do so and represents the fifth generation <strong>of</strong> a line <strong>of</strong> <strong>Pitt</strong> physiciansdating back to Lester Haven Bodkin, who enrolled in 1886 as part <strong>of</strong> the firstclass to enter <strong>Pitt</strong> med’s forerunner, the Western Pennsylvania <strong>Med</strong>ical College.Robert and the other Tisherman children have always been surrounded by medicine.When child-care arrangements fell through, Susan says, they put the childrenin an <strong>of</strong>fice behind the ER. “They splinted each other. … My kids learned to buildtrauma packs from the time they were 5 and 6 years old.”For those who aren’t in the know, the family ties can cause confusion. A col-36 PITTMED
RIGHT: Sun. BELOW: Asare (right) at theU.S. Capitol last year with formerU.S. Congressman Louis Stokes.<strong>of</strong> interventional cardiologyresearch and education atthe <strong>University</strong> <strong>of</strong> Chicago<strong>Med</strong>ical Center, is amonga small group <strong>of</strong> cardiologistswho routinely performcoronary angiographies—to test heart health—via the radialartery in the wrist, rather than the femoral artery in the leg.Though the transradial technique is used in less than 10percent <strong>of</strong> angiographies performed in the United States,Nathan and others believe it can cut costs, allow for earlierdischarges, and reduce patient discomfort. He has taughtthe technique at national symposia and workshops andhopes that it will soon be a more widely practiced procedure.“There is a learning curve and some associated growingpains, but the potential pay<strong>of</strong>f is huge,” he says.’00s It’s a common refrain in legislation:Health care policymakers <strong>of</strong>ten have little to no experiencein health care themselves. But Akua G. Asare (MD ’06)set out to change that as the 2011 American PsychiatricAssociation Jeanne Spurlock, MD, Congressional Fellow.Last year, Asare put her dual training in general medicineand psychiatry to work on a nu<strong>mb</strong>er <strong>of</strong> policy <strong>issue</strong>s rangingfrom mental health to antimicrobial-resistant pathogens.She first became interested in health care policy during herresidency in psychiatry at the <strong>University</strong> <strong>of</strong> Miami LeonardM. Miller School <strong>of</strong> <strong>Med</strong>icine/Jackson Memorial Hospital,when Asare was involved with the Committee <strong>of</strong> Internsand Residents, a national house staff union that advocatesfor better working conditions in health care. Though theSpurlock Fellowship kept her from practicing medicine,she says, “It’s opened up a different world wherein I canbecome a change agent on a macro scale.”LEFT: The recently deceased surgeon, Lester Dunmire. RIGHT:Two more <strong>Pitt</strong> med generations—Robert Tisherman is flankedby his parents, Samuel Tisherman and Susan Dunmire.Smell tests—where people measure their abilityto recognize distinct scents—have been featuredon The Dr. Oz Show as simple and effective indicators<strong>of</strong> your risk <strong>of</strong> developing Alzheimer’s disease.Finding evidence <strong>of</strong> this to be lacking, Gordon Sun(MD ’06) sought to sniff out the truth. The Robert WoodJohnson Foundation clinical scholar at the <strong>University</strong> <strong>of</strong>Michigan reexamined the research behind such testsand published a paper this May in Laryngoscope, whichcaught the attention <strong>of</strong> WebMD and <strong>Med</strong>ical NewsToday. His conclusion? “The evidence is not strongenough to support the regular use <strong>of</strong> smell tests in predictingthe development <strong>of</strong> Alzheimer’s dementia.” Henotes the correlation between decreased olfaction andAlzheimer’s; however, rigorous studies are still neededbefore smell tests can be used to predict onset <strong>of</strong> thedisease, he says.’10s In<strong>Med</strong>ical Association Foundation awarded Alana OttoAugust 2011, the American(MD ’12) a $10,000 Physicians <strong>of</strong> Tomorrow scholarship.Otto was one <strong>of</strong> only 18 to be presented with thehonor last year. The foundation scholarships are givenannually to medical students who not only demonstrateexcellence within the classroom, but also have shown acommitment to community involvement and volunteering.A regular volunteer with the Allegheny ReproductiveHealth Center and the Women’s Center and Shelter <strong>of</strong>Greater <strong>Pitt</strong>sburgh, Otto started her pediatrics residencyin June at Northwestern <strong>University</strong>/Lurie Children’sHospital in Chicago. She has also served as a mentor forpregnant teenagers around <strong>Pitt</strong>sburgh. Otto says shelooks forward to “the opportunity to be an influentialme<strong>mb</strong>er <strong>of</strong> patients’ lives and communities in additionto providing medical care.” As a pediatrician, she wantsto make “health care more accessible, relevant, andeffective for children.”—Hayavadhan Thuppal and Elaine Vitoneleague <strong>of</strong> Sam’s wasonce approached by astudent worried aboutan affair. When pressedfor evidence, the studentrevealed seeingPr<strong>of</strong>essors Dunmire andTisherman holding hands.The amused colleagueresponded, They’ve beenmarried for 20 years!Another time, Susanreports, she was “talking back” to Sam in the ER: “A resident turned to me and said,‘You can’t talk to him that way—he’s the attending for surgery.’ And I said, ‘Yes I can;he’s my husband!’”Robert shares dinner with his parents and sometimes stops by his mom’s <strong>of</strong>fice todrop <strong>of</strong>f a c<strong>of</strong>fee. Eventually, he’ll take classes taught by his parents, which will, inall likelihood, generate more entertaining stories. —Em MaierTHE WAY WE ARECLASS OF ’88When Elaine Hylek (MD ’88) thinksabout her days at the School <strong>of</strong><strong>Med</strong>icine, she rattles <strong>of</strong>f the namesand residencies <strong>of</strong> her classmates as if shegraduated yesterday. Hylek was the classpresident her first two years and got to knowmany <strong>of</strong> her classmates through a nu<strong>mb</strong>er <strong>of</strong>events: the hayride, the ’50s-themed dance,the Scope and Scalpel performances. Today,Hylek is a world-renowned expert in theprevention <strong>of</strong> stroke and atrial fibrillation,as well as the safety <strong>of</strong> anticoagulants. She isalso the director <strong>of</strong> the Thro<strong>mb</strong>osis Serviceat Boston <strong>Med</strong>ical Center, associate director<strong>of</strong> the Education and Training Division <strong>of</strong>the Boston <strong>University</strong> Clinical TranslationalScience Institute, and pr<strong>of</strong>essor <strong>of</strong> medicineat Boston <strong>University</strong>.Hylek recalls more than just thename and residency <strong>of</strong> fellow <strong>Pitt</strong> mederEric Peterson (MD ’88)—she regularlycollaborates with him. Peterson is director<strong>of</strong> the Duke Clinical Research Instituteand pr<strong>of</strong>essor <strong>of</strong> medicine in the Division<strong>of</strong> Cardiology at Duke <strong>University</strong> <strong>Med</strong>icalCenter. He studies outcomes research andcontemporary management <strong>of</strong> acute coronarysyndromes.MosessoAnother classmateHylek reme<strong>mb</strong>ers fondlyis Vincent Mosesso(MD ’88, Emergency<strong>Med</strong>icine Resident ’91).The two grew up togetheron Brinwood Avenue inBaldwin before reunitingat the School <strong>of</strong> <strong>Med</strong>icine.Mosesso is pr<strong>of</strong>essor <strong>of</strong> emergencymedicine at <strong>Pitt</strong>, medical director<strong>of</strong> prehospital care for UPMC, and medicaldirector <strong>of</strong> the Sudden Cardiac ArrestAssociation, a nonpr<strong>of</strong>it that promotesawareness and treatment <strong>of</strong> sudden cardiacarrest, as well as support for survivors.As a med student, Mosesso performedin Scope and Scalpel with Hylek … sort <strong>of</strong>.“My singing was deemed so bad that I hadto lip sync, and another classmate sang fro<strong>mb</strong>ackstage,” he says.Husband-and-wife alums Ken Washburn(MD ’88) and Anne Logan (MD ’88) metFALL 2012 37
Washburn and Loganat <strong>Pitt</strong> med. Logan, a pediatrician, hasa private practice in Boerne, a TexasHill Country town the couple has calledhome since 2000. Washburn is the ValeroDistinguished Chair in Transplantationand director <strong>of</strong> liver transplantation at the<strong>University</strong> <strong>of</strong> Texas Health Science Centerat San Antonio. An expert in pediatricand adult liver transplantation, he’s leda nu<strong>mb</strong>er <strong>of</strong> clinical research protocolsin the field. During the past decade,Washburn has also served on numerousstate, regional, and national committeesthat match organ donorsand recipients.For Albert Faro (MD’88, Pediatrics Resident ’91,Pulmonary <strong>Med</strong>icine Fellow’96), studying and trainingin <strong>Pitt</strong>sburgh helped himdiscover his “two loves”—Farocaring for patients withcystic fibrosis (CF) and transplantmedicine. (Nearly half <strong>of</strong> pediatriclung transplant recipients have CF.) Asthe first president <strong>of</strong> the InternationalPediatric Lung Transplant Collaborative,he brought together lung transplant centersfrom around the world to better servepatients in need. Faro continues his academicand clinical work at the Washington<strong>University</strong> School <strong>of</strong> <strong>Med</strong>icine in St.Louis, where he is associate pr<strong>of</strong>essor <strong>of</strong>pediatrics. —HTCHARLES E. COPELANDNOV. 16, 1931–MAY 8, 2012Charles E. Copeland (MD ’58, GeneralSurgery Resident ’63, American CancerSociety Fellow ’65, Vascular Surgery andTransplantation Research Fellow ’66) was wellknown for founding UPMC Mercy’s burn unit,the first <strong>of</strong> its kind in Pennsylvania, in 1967.He remained a constant presence there until sixmonths before he died <strong>of</strong> liver cancer in May. Butthe 80-year-old’s “biggest legacy” is the surgicaltraining program at Mercy, says surgeon HarryW. Sell Jr., a longtime colleague who followedCopeland as the chair <strong>of</strong> Mercy’s Department<strong>of</strong> Surgery. Copeland, a general, vascular, andtrauma surgeon and clinical pr<strong>of</strong>essor <strong>of</strong> surgeryat <strong>Pitt</strong>, was among the first local doctors trainedin advanced trauma life support in the 1960sand continued teaching residents at Mercy forsome 50 years. Apart from a one-year fellowshipin Boston, Copeland spent his <strong>entire</strong> career in<strong>Pitt</strong>sburgh.It has not been uncommon for formerpatients to call the burn unit asking to talk toCopeland decades after being in his care. Sellrecalls Copeland as the sort <strong>of</strong> surgeon who gavehimself <strong>entire</strong>ly to his pr<strong>of</strong>ession and his family.“That kind <strong>of</strong> person doesn’t come along too<strong>of</strong>ten,” says Sell. “He had a tremendous amount<strong>of</strong> accomplishments here, and you couldn’t tell.He was hu<strong>mb</strong>le about it.” —HTPEARL G. MC NALLFEB. 3, 1923–MARCH 26, 2012As an anesthesiologist, Pearl McNall (MD’49, Intern ’50, Anesthesiology Resident’52) was known for her precise manner,take-charge attitude, and lifelong devotion totreating patients with myasthenia gravis. She diedMarch 26, at age 89, <strong>of</strong> congestiveheart failure.“She was the most meticulous,careful anesthesiologist on mystaff,” says Rick Siker, an MD,longtime friend, and former director<strong>of</strong> anesthesiology at Mercy. “ICopelandwould always assign new residents tohave at least one week’s rotation withDr. McNall. She would teach themhow important it was to be meticulouslycareful with patient safety.”She is also fondly reme<strong>mb</strong>ered,Siker says, for once telling a too-curtsurgeon who demanded she come toMcNallthe hospital late one night, “You’vegot to wait until I get my girdle on.”“She was well known for that comment,” Sikersays. “One could not dictate to Dr. McNall, andI think it was one <strong>of</strong> her finest qualities.” McNallwas the first woman appointed to Mercy’s seniormedical staff, a position she accepted hu<strong>mb</strong>ly.As a young physician, McNall developed anearly interest in studying and treating myastheniagravis, a chronic neuromuscular disease thatcauses muscle weakness. With Francis Foldes,an MD and c<strong>of</strong>ounder <strong>of</strong> the MyastheniaGravis Clinic <strong>of</strong> Western Pennsylvania at MercyHospital, McNall authored numerous studieson myasthenia gravis treatments and protocol,as well as new localized muscle relaxants inanesthesiology. McNall became a physician atthe clinic in 1957 and in 1962 was appointedits director. She later served as chair <strong>of</strong> theMyasthenia Gravis Foundation <strong>of</strong> America andreceived its Outstanding Woman <strong>of</strong> the YearAward in 1974.Outside <strong>of</strong> the hospital, McNall was anardent bridge player, world traveler, and botanist.Her family asks that donations be made onher behalf to the Myasthenia Gravis Association<strong>of</strong> Western Pennsylvania, to which she remaineddevoted throughout her career and late in life.—Jenelle Pifer’30sTHOMAS M. BALDWIN SR.MD ’37MAY 2, 2012PAUL M. RIKEMD ’38MAY 10, 2012’40sCARL BENNETT BEANRES ’44JULY 14, 2012IN MEMORIAMLESTER DUNMIREMD ’48JULY 21, 2012’50sJOSEPH G. BURGERMD ’55JULY 2, 2012JAMES ANTHONY ROCKMD ’56JUNE 1, 2012’60sNOEL JOHN GILLETTEMD ’60MARCH 22, 2012DAVID STELLEMD ’61JUNE 27, 2012JOHN R. MISAGEMD ’62MAY 4, 2012JOSEPH BRENNERMD ’65JULY 30, 2012LORETTA R. LOEBMD ’57, RES ’68MAY 11, 2012’80sGERALD BRETT WEISSMD ’87JUNE 2, 2012GREGORY S. SHEFFOMD ’89MAY 27, 201238 PITTMED
WE KNEW YOU WHENCHARLES COCHRANEAND THEPITTSBURGH FIVEBY JENELLE PIFEROn the afternoon <strong>of</strong> March 6, 2012, CharlesCochrane sat nervously near the phone inhis lab at the Scripps Research Institute inLa Jolla, Calif. “You’re waiting, waiting, waiting,”says the 81-year-old MD and former <strong>Pitt</strong> pr<strong>of</strong>essor<strong>of</strong> pathology. The call he was expecting fromthe FDA was one that would determine the fate<strong>of</strong> a project he’d labored on for 25 years. The newdrug he’d created was called Surfaxin, a syntheticsurfactant that coats the lungs <strong>of</strong> preterm infantsin respiratory distress and helps them breathe.Cochrane knew the potential was enormous.The phone rang. A colleague spoke: “Charley,in 2 seconds you’re going to be very happy.” Itwas the team’s fifth attempt, and Surfaxin wasfinally approved. The drug will be on the marketthis October.In 1956, at 25, Cochrane joined a group <strong>of</strong>up-and-coming pathology researchers at <strong>Pitt</strong>.“Our department was always rated the best by thestudents because we were such young cowboys,”Cochrane says.“He displayed a lot <strong>of</strong> enthusiasm. That resonatedwith some students,” says Richard Trackler(MD ’61). “They thought, He’s more like we are.”In 1961 Cochrane decided to pursue researchfull time. He and four others from <strong>Pitt</strong>—thendepartment-chairFrank Dixon, Joseph Feldman,BRIAN KLATT (MD ’97)PresidentJAN MADISON (MD ’85)President-electMARGARET LARKINS-PETTIGREW (MD ’94)SecretaryPETER FERSON (MD ’73)TreasurerROBERT E. LEE (MD ’56)HistorianSUSAN DUNMIRE (MD ’85)Executive DirectorMEDICAL ALUMNIASSOCIATION OFFICERS1961 HIPPOCRATEAN<strong>Pitt</strong> med’s Department <strong>of</strong> Pathology in the 1961 Hippocratean. That year, the“<strong>Pitt</strong>sburgh Five”—William Weigle, Charles Cochrane (top row, fifth and sixth fromthe left, respectively), Joseph Feldman, Frank Dixon (front row, third and fourthfrom the left, respectively), and Jacinto Vazquez (not pictured)—left <strong>Pitt</strong>sburgh forCalifornia, establishing the forerunner <strong>of</strong> today’s Scripps Research Institute.DONALD MRVOS (MD ’55)CARL ROBERT FUHRMAN (MD ’79)GREGORY M. HOYSON (MD ’82)SAM TISHERMAN (MD ’85)JOHN F. MAHONEY (MD ’90)ADAM GORDON (MD ’95)CHARISSA B. PACELLA (MD ’98)HEATHER HEINRICHS WALKER (MD ’99)BRETT PERRICELLI (MD ’02)Me<strong>mb</strong>ers at LargeM-200k Scaife Hall<strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh<strong>Pitt</strong>sburgh, PA 15261tel 412-648-9090; fax 412-648-9500medalum@medschool.pitt.eduJacinto Vazquez, and William Weigle, a soughtaftergroup called the “<strong>Pitt</strong>sburgh Five”—moved to California to a then-unknown complexcalled the Scripps Research Institute,where an enterprising hospital director promisedto build them a facility and leave themalone. Dixon eventually became the institute’sfirst director and led the research program fortwo-and-a-half decades.In 1988, a pediatrician studying the humansurfactant—which coats the air sacs in thelungs and helps keep them open—came toCochrane for help. His team had identified thelipids involved but didn’t quite understand itsproteins. Cochrane set about isolating them.“The first thing that struck me was that inour columns for separating the proteins therewas precipitation at the top,” says Cochrane.Until then, no protein in the human body wasknown to be insoluble in water. “I couldn’tbelieve what I was seeing,” says Cochrane, nowpr<strong>of</strong>essor emeritus in Scripps’ Department <strong>of</strong>Immunology and Microbial Science.He analyzed the DNA, determined the<strong>entire</strong> sequence <strong>of</strong> the protein, and pinpointedwhich regions were responsiblefor the surfactant activity.Knowing this, he made a verysimple version <strong>of</strong> the protein,mixed it back in with syntheticlipids, and lo and behold: He’dmade an <strong>entire</strong>ly synthetic surfactant.CochraneInfant respiratory distress syndrome is alife-threatening disorder that occurs when prematurelungs don’t have the proper elasticityto spring back open after exhaling. Surfactantsrestore the stretch and enable these babies tobreathe on their own. Until now, they typicallyhave been given surfactants derived from animallungs, but Surfaxin has been demonstratedto work equally well, says Jennifer Kloesz, <strong>Pitt</strong>associate pr<strong>of</strong>essor <strong>of</strong> pediatrics and clinicaldirector <strong>of</strong> the neonatal intensive care unit atMagee-Womens Hospital <strong>of</strong> UPMC. Surfaxineliminates risks <strong>of</strong> impurities inherent in animalproducts. “And sometimes different religiousor cultural groups have concerns with ususing animal-derived products,” Kloesz says.Getting approval from the FDA was along and turbulent process in part becausethe drug type was so new, Cochrane says. He’snow working on an aerosol delivery method,so Surfaxin can be administered in developingcountries without ventilators. He’s alsoconfident the drug will find new applications,treating diseases like cystic fibrosis and acuteasthma in adults.The four other me<strong>mb</strong>ers <strong>of</strong> the <strong>Pitt</strong>sburghFive have since died, and colleagues at Scrippsnow jokingly call Cochrane “the last <strong>of</strong> theMohicans.”“There is something almost mythical aboutthe <strong>Pitt</strong>sburgh Five,” says Trackler. “They werethe nucleus <strong>of</strong> the Scripps Research Institute,these cowboys.”FALL 2012 39
LAST CALLTHAT’S NOT NATURALIt would be surprising to find a display about E. coli x1776 in anatural-history style exhibit. For one thing, it’s a bacterium—notexactly the usual dinos-and-Darwin fare. More importantly, it’snot natural. E. coli x1776 was invented in a laboratory in 1976 asa safe research organism that couldn’t survive outside the lab.Genentech c<strong>of</strong>ounder Herbert Boyer (PhD ’63) used E. coli x1776in the work that led to mass-produced insulin; his invention <strong>of</strong>reco<strong>mb</strong>inant DNA could be seen as a Promethean moment. Butwhile a lab origin may take it out <strong>of</strong> the running for natural historymuseums, it makes E. coli x1776 perfect for the Center forPostNatural History.Founded by Richard Pell, associate pr<strong>of</strong>essor in Carnegie Mellon<strong>University</strong>’s School <strong>of</strong> Art, with colleagues including lead scientificadvisor Lauren Allen, a graduate student in <strong>Pitt</strong>’s Science andEducation Policy Program, the center is an archive and public-outreachproject that features living organisms that have been alteredby humans. From sterilized screwworms to transgenic chestnut trees(engineered to resist chestnut blight), the center keeps documentationand specimens <strong>of</strong> transgenic living things. It also createsexhibits for its new storefront gallery in <strong>Pitt</strong>sburgh’s Garfield neighborhoodand for venues as diverse as European avant-garde artspaces and the Smithsonian Museum <strong>of</strong> Natural History, where Pellwas a research fellow in 2010.—Justin Hopper—Illustration courtesy Center for PostNatural History40 PITTMED
C A L E N D A ROF SPECIAL INTEREST TO ALUMNI AND FRIENDSINDIANAPOLISHEALTH SCIENCESALUMNI RECEPTIONSEPTEMBER 276 p.m.Indianapolis, Ind.MUSGRAVELECTURESHIPOCTOBER 12–13J. William Futrell, MD, SpeakerHOUSTONHEALTH SCIENCESALUMNI RECEPTIONJANUARY 17, 20136 p.m.Houston, TexasAUSTINHEALTH SCIENCESALUMNI RECEPTIONJANUARY 18, 20136 p.m.Austin, TexasPALM BEACHHEALTH SCIENCESALUMNI RECEPTIONFEBRUARY 13, 20136 p.m.Palm Beach, Fla.2013 WINTER ACADEMYFEBRUARY 15, 2013Ritz-CarltonNaples, Fla.www.winteracademy.pitt.eduMEDICAL ALUMNIWEEKEND 2013MAY 17–20, 2013Reunion Classes:2003 19981993 19881983 19781973 19681963 1958UPCOMINGHEALTH SCIENCESALUMNI RECEPTIONSDATES TBASt. Augustine, Fla.Atlanta, Ga.Phoenix, Nev.Los Angeles, Calif.For information on an event,unless otherwise noted, contactPat Carver, 412-647-5307,cpat@pitt.eduTO FIND OUT WHAT ELSE IS HAPPENING AT THE MEDICAL SCHOOL, GO TO www.health.pitt.edu
UNIVERSITY OF PITTSBURGHSCHOOL OF MEDICINESUITE 401 SCAIFE HALLPITTSBURGH, PA 15261CHANGE SERVICE REQUESTEDNONPROFIT ORG.U.S. POSTAGEPAIDPITTSBURGH, PAPERMIT NO. 511THE OWL, 1955THE GIFT THAT GIVES BACKWhen you establish a charitable gift annuity (CGA) with the<strong>University</strong> <strong>of</strong> <strong>Pitt</strong>sburgh School <strong>of</strong> <strong>Med</strong>icine, you receive an incometax deduction and an annual payment for life. Deferred CGAs giveyou the option to defer the income payments so that you receive agreater fixed income later. At the time <strong>of</strong> your death, the funds youcontributed when you established the CGA will benefit the schoolor a specific program you have designated. The examples below arebased on a gift <strong>of</strong> $10,000.If you would like to learn more about theways in which you can arrange for yourlegacy to the School <strong>of</strong> <strong>Med</strong>icine, contact:Lisa J. SciulloForbes Tower, Suite 80843600 Forbes Ave.<strong>Pitt</strong>sburgh, PA 15213412-647-0515slisa@PMHSF.orgwww.pitt.planyourlegacy.orgOwing to varying restrictions, <strong>Pitt</strong> is notable to <strong>of</strong>fer gift annuities in some states.AGE RATE YEARLY ESTIMATEDINCOME DEDUCTION55 4.0 $400 $1,85160 4.4 $440 $2,23165 4.7 $470 $2,95670 5.1 $510 $3,69175 5.8 $580 $4,26880 6.8 $680 $4,79185 7.8 $780 $5,50290 9.0 $900 $6,190


![entire issue [pdf 12.7 mb] - Pitt Med - University of Pittsburgh](https://img.yumpu.com/44997419/1/500x640/entire-issue-pdf-127-mb-pitt-med-university-of-pittsburgh.jpg)
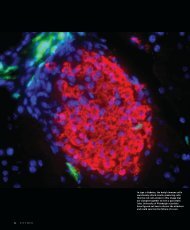

![entire issue [pdf 2.79 mb] - Pitt Med - University of Pittsburgh](https://img.yumpu.com/50435398/1/190x231/entire-issue-pdf-279-mb-pitt-med-university-of-pittsburgh.jpg?quality=85)
![entire issue [pdf 6.47 mb] - Pitt Med - University of Pittsburgh](https://img.yumpu.com/50360689/1/190x231/entire-issue-pdf-647-mb-pitt-med-university-of-pittsburgh.jpg?quality=85)
![entire issue [pdf 12.7 mb] - Pitt Med - University of Pittsburgh](https://img.yumpu.com/49831615/1/190x231/entire-issue-pdf-127-mb-pitt-med-university-of-pittsburgh.jpg?quality=85)







![entire issue [pdf 11.3 mb] - Pitt Med - University of Pittsburgh](https://img.yumpu.com/46685830/1/190x231/entire-issue-pdf-113-mb-pitt-med-university-of-pittsburgh.jpg?quality=85)