Chapter 17 Practice Problems: Acid-Base Titrations and Buffers
Chapter 17 Practice Problems: Acid-Base Titrations and Buffers
Chapter 17 Practice Problems: Acid-Base Titrations and Buffers
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
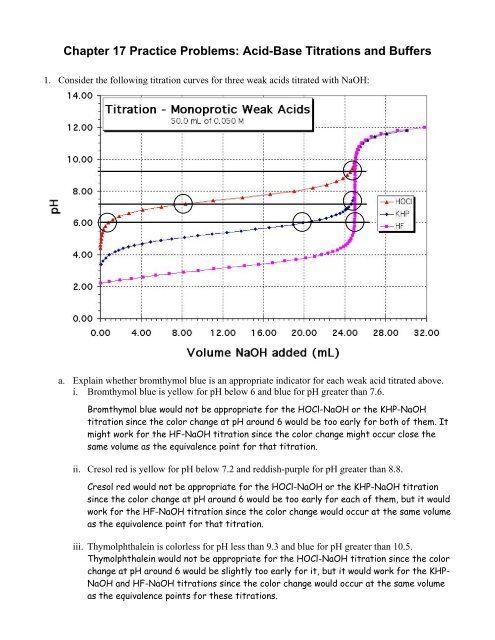
<strong>Chapter</strong> <strong>17</strong> <strong>Practice</strong> <strong>Problems</strong>: <strong>Acid</strong>-<strong>Base</strong> <strong>Titrations</strong> <strong>and</strong> <strong>Buffers</strong>1. Consider the following titration curves for three weak acids titrated with NaOH:a. Explain whether bromthymol blue is an appropriate indicator for each weak acid titrated above.i. Bromthymol blue is yellow for pH below 6 <strong>and</strong> blue for pH greater than 7.6.Bromthymol blue would not be appropriate for the HOCl-NaOH or the KHP-NaOHtitration since the color change at pH around 6 would be too early for both of them. Itmight work for the HF-NaOH titration since the color change might occur close thesame volume as the equivalence point for that titration.ii. Cresol red is yellow for pH below 7.2 <strong>and</strong> reddish-purple for pH greater than 8.8.Cresol red would not be appropriate for the HOCl-NaOH or the KHP-NaOH titrationsince the color change at pH around 6 would be too early for each of them, but it wouldwork for the HF-NaOH titration since the color change would occur at the same volumeas the equivalence point for that titration.iii. Thymolphthalein is colorless for pH less than 9.3 <strong>and</strong> blue for pH greater than 10.5.Thymolphthalein would not be appropriate for the HOCl-NaOH titration since the colorchange at pH around 6 would be slightly too early for it, but it would work for the KHP-NaOH <strong>and</strong> HF-NaOH titrations since the color change would occur at the same volumeas the equivalence points for these titrations.
. Calculate the molarity for the NaOH used in the titrations above if 50.0 mL of each acid wasused, <strong>and</strong> all the acids had a molarity of 0.050M.0.050 mol HA 1 mol HA 1L0.0500 L××× = 0.10M NaOHL 1 mol NaOH 0.0250 Lc. Indicate the major species at the equivalence point for each weak acid-NaOH titration shownabove, then write the relevant dissociation or hydrolysis reaction for the species that regulates thepH at the equivalence point.i. major species: OCl − reaction: OCl − (aq) + H 2 O(l) HOCl(aq) + OH − (aq)ii. major species: Phthalate − reaction: Phth −2 (aq) + H 2 O(l) HPhth − (aq) + OH − (aq)iii. major species: F − reaction: F − (aq) + H 2 O(l) HF(aq) + OH − (aq)d. Explain whether the following solutions will be acidic, basic, or neutral at the equivalence pointby indicating the major species that regulates the pH for each solution:i. a strong acid titrated with a strong baseiv. a strong base titrated with a strong acidThe solution would be neutral since the conjugate base of a strong acid is a weakerbase than water, so the major species (conjugate base of strong acid <strong>and</strong> cation ofstrong base) remaining in solution are neutral.ii. a weak acid titrated with a strong baseThe solution would be basic since the conjugate base of a weak acid is a stronger basethan water, so it hydrolyzes in water to produce hydroxide ions.iii. a weak base titrated with a strong acidThe solution would be acidic since the conjugate acid of a weak base dissociates inwater to produce H + (or hydronium) ions.