CH CH2 CH2 CH2 CH2 CH2 CH3 CH3 - yourhomework.com Home ...
CH CH2 CH2 CH2 CH2 CH2 CH3 CH3 - yourhomework.com Home ...
CH CH2 CH2 CH2 CH2 CH2 CH3 CH3 - yourhomework.com Home ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
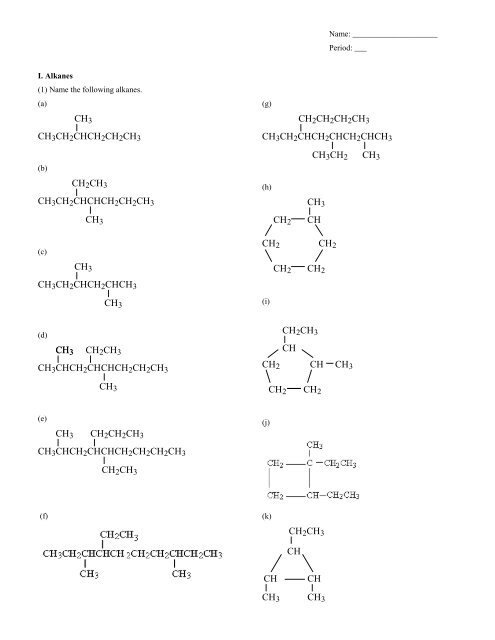
Name: _____________________Period: ___I. Alkanes(1) Name the following alkanes.(a)(g)<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3(b)(c)(d)(e)<strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 3(h)<strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2<strong>CH</strong> 2 <strong>CH</strong> 2(i)<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> <strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 2(j)<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong> 3(f)(k)<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong><strong>CH</strong><strong>CH</strong> 3<strong>CH</strong><strong>CH</strong> 3
(2) Draw the following alkanes.(a) 2-methylpropane(g) 3-ethyl-2-methyl-5,6-dipropylnonane(b) 3-ethyl-2-methylhexane(h) 1-ethylcyclopropane(c) 2,2-dimethylbutane(i) 2,3-dimethyl-1-propylcyclobutane(d) 3-ethyl-2,5-dimethylheptane(j) 1,2-diethyl-3-methylcyclopentane(e) 3,3,5-trimethyloctane(k) 1,3-dimethylcyclohexane(f) 5-butyl-2,3,6-trimethyldecane(3) Name and draw the five structural isomers of C 6 H 14 .
Name: _____________________Period: ___II. Alkenes(1) Name the following alkenes.(d)(a)<strong>CH</strong> 2<strong>CH</strong> <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong>=<strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong>(b)<strong>CH</strong> 3 <strong>CH</strong>=<strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 3(e)<strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong><strong>CH</strong><strong>CH</strong> 3<strong>CH</strong> 3(c)<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong>=<strong>CH</strong><strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong><strong>CH</strong>=<strong>CH</strong><strong>CH</strong> 3<strong>CH</strong> 3(f)<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 2<strong>CH</strong><strong>CH</strong><strong>CH</strong><strong>CH</strong> 2<strong>CH</strong> 2(2) Draw the following alkenes.(a) 4-Methyl-2-hexene(d) 1-methylcyclopentene(b) 3-Ethyl-4-methyl-2-pentene(e) 6-Ethyl-3,3-dimethyl-4-nonene(c) 3-Ethyl-2,4-dimethyl-3-heptene(f) 3-Ethyl-4,5-dimethylcyclohexene
(3) (a) Name the two stereoisomers of 2-hexene.#1 #2<strong>CH</strong> 3 H<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3HC=C<strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3HC=<strong>CH</strong>(b) Name and draw the two stereoisomers of 3-hexene.III. Alkynes(1) Name the following alkynes.(a) <strong>CH</strong> 3 CC<strong>CH</strong> 2 <strong>CH</strong> 3(c)<strong>CH</strong> 3(b)<strong>CH</strong> 3 C = CC<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 C = C<strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 3(d)<strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 C = C<strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3(2) Draw the following alkynes.(a) 4-Ethyl-2-heptyne(c) 2,5,6-Trimethyl-3-octyne(b) 3-Ethyl-5-methyl-1-hexyne(d) 3,8-Dimethyl-5-decyne
Answers:I. Alkanes(c)(d)<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong>(1) (a) 3-Methylhexane3 <strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 3(b) 3-Ethyl-4-methylheptane<strong>CH</strong> 3 <strong>CH</strong> 3(c) 2,4-Dimethylhexane(e)<strong>CH</strong>(d) 4-Ethyl-2,5-dimethyloctane2 <strong>CH</strong><strong>CH</strong>(e) 5-Ethyl-2-methyl-4-propylnonane3(j)(f) 4-Ethyl-3,8-dimethyldecane <strong>CH</strong> 3 <strong>CH</strong> 2 C<strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3(g) 4,6-Diethyl-2-methyldecane<strong>CH</strong> 3 <strong>CH</strong> 3<strong>CH</strong>(h) 1-Methylcyclohexane(i) 1-Ethyl-2-methylcyclopentane (f)<strong>CH</strong> 2(j) 1,2-Diethyl-1-methylcyclobutane(k) 1-Ethyl-2,3-dimethylcyclopropane<strong>CH</strong> 2 <strong>CH</strong>(k)(2) (a)(g)<strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3(b)<strong>CH</strong> 2 <strong>CH</strong> <strong>CH</strong> 33 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong>(h)2<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong>(i)<strong>CH</strong> <strong>CH</strong><strong>CH</strong> <strong>CH</strong> <strong>CH</strong> 3<strong>CH</strong><strong>CH</strong> <strong>CH</strong><strong>CH</strong> 3 <strong>CH</strong> 32 32 2<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2<strong>CH</strong> 2<strong>CH</strong> 3<strong>CH</strong><strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 2(3)1. Hexane<strong>CH</strong> 3 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 33. 3-Methylpentane<strong>CH</strong> 35. 2,2-Dimethylbutane<strong>CH</strong> 3<strong>CH</strong>2. 2-Methylpentane3 <strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong>4. 2,3-Dimethylbutane3<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 C<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong><strong>CH</strong><strong>CH</strong> 3<strong>CH</strong> 3
II. Alkenes(1) (a) 3-hexene(c)(b) 4-Ethyl-2-heptene<strong>CH</strong> 3 <strong>CH</strong> 3(e)(c) 4,5-Dimethyl-2-hexene(d) 3,5-Dimethylcyclopentene(e) 2,6-Dimethyl-5-propyl-3-octene(f) 3-Ethylcyclohexene(2)(a)(b)<strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong>C=C<strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 3(d)<strong>CH</strong> 2<strong>CH</strong> 2 <strong>CH</strong> 2C <strong>CH</strong><strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 2<strong>CH</strong><strong>CH</strong> <strong>CH</strong> 3<strong>CH</strong>(f)<strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong><strong>CH</strong><strong>CH</strong> 3 <strong>CH</strong>=C<strong>CH</strong><strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong> 2 C<strong>CH</strong>=<strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 2(3) (a) #1: trans-2-hexene (or (E)-2-hexene)#2: cis-2-hexene (or (Z)-2-hexene)(b) trans-3-hexene (or (E)-3-hexene)cis-3-hexene (or (Z)-3-hexene)III. Alkynes(1) (a) 2-pentyne(b) 5-Methyl-3-heptyne(c) 4,4-Dimethyl-2-hexyne(d) 5-Ethyl-6-methyl-3-octyne(b)<strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> = C<strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong><strong>CH</strong> 3(d)(a)<strong>CH</strong> 2 <strong>CH</strong> 3(c)<strong>CH</strong> 3<strong>CH</strong> 3 C = C<strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3 <strong>CH</strong>C = C<strong>CH</strong><strong>CH</strong><strong>CH</strong> 2 <strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3<strong>CH</strong> 3
HydrocarbonsName: _____________________Period: ___(1) Classify each hydrocarbon as an alkane, alkene, or alkyne. Give the name for the molecule.(a)(f)(b)(g)(c)(h)(d)(i)(e)(j)(2) Classify each hydrocarbon as an alkane, alkene, or alkyne. Draw the molecule.(a) 2,2,4,4–tetramethylpentane(f) 3–ethyl–4–methyl–1–octene(b) 2–methyl–1–butene(g) 3,5–diethylcyclopentene(c) 1–nonyne(h) 1,2,3–trimethylcyclohexane(d) 1,2–dipropylcyclohexane(i) 4–methyl–2–heptyne
(e) 5–butyldecane(j) 1,2-dimethylcyclobutene(3) Give the formula and draw two different structures for the molecule benzene.(4) (a) The molecule 1–methylbenzene is also called “toluene”. Draw the structure for toluene.(b) The molecule 1–ethylbenzene is also called phenylethane. Draw the structure for this molecule.(5) A benzene molecule with two methyl groups is called xylene. The molecule is given a name depending on the location of themethyl groups.(a) The molecule 1,2–dimethylbenzene is also called “ortho–xylene”. Draw the structure for ortho–xylene.(b) The molecule 1,3–dimethylbenzene is also called “meta–xylene”. Draw the structure for meta–xylene.(c) The molecule 1,4–dimethylbenzene is also called “para–xylene”. Draw the structure for para–xylene.(6) Give the name and drawing for the two structural isomers for C 3 H 6 .
Answers:(1)(a) 4–propylnonane(b) 2–methyl–3–octene(c) 5–ethyl–2–heptyne(d) 4,4–diethyloctane(e) 2–ethyl–3–methyl–1–propylcyclobutane(2)(a)(f) 1, 2–diethylcyclopropane(g) 2,3,8,9–tetramethyl–5–decyne(h) 3–ethyl–5–methylcyclopentene(i) 2,5–dimethyl–3–hexyne(j) 3,3,7–trimethylnonane(f)(b)(g)(c)(h)(d)(i)(e)(j)
(3) C 6 H 6 (4) (a) (b)(5)(a) (b) (b)(6)cyclopropane1–propene