10826 AP Chemistry Chemical Equations Worksheet
10826 AP Chemistry Chemical Equations Worksheet
10826 AP Chemistry Chemical Equations Worksheet
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
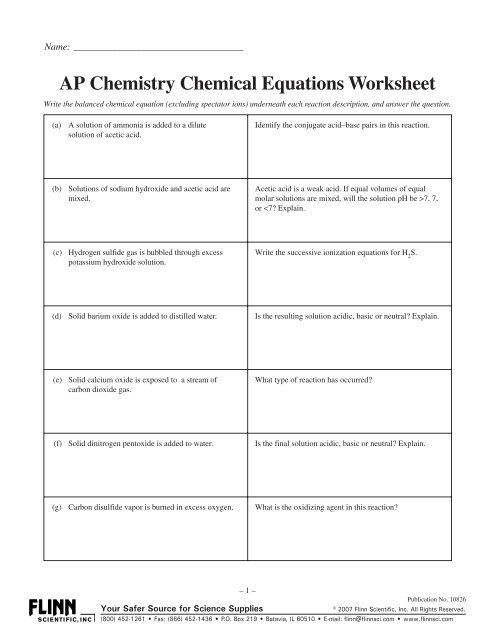
Name: ___________________________________<strong>AP</strong> <strong>Chemistry</strong> <strong>Chemical</strong> <strong>Equations</strong> <strong>Worksheet</strong>Write the balanced chemical equation (excluding spectator ions) underneath each reaction description, and answer the question.(a) A solution of ammonia is added to a dilutesolution of acetic acid.Identify the conjugate acid–base pairs in this reaction.(b) Solutions of sodium hydroxide and acetic acid aremixed.Acetic acid is a weak acid. If equal volumes of equalmolar solutions are mixed, will the solution pH be >7, 7,or
(h) Lithium metal is burned in air.Besides combustion, what type of reaction could this beclassified as?(i) A solution of diamminesilver(I) chloride is treatedwith dilute nitric acid.What is the driving force for this reaction?(j) A concentrated solution of ammonia is added to asuspension of zinc hydroxide.What are the possible molecular geometries for the complexion product?(k) Excess concentrated sodium hydroxide solution isadded to solid aluminum hydroxide.Name any complex ion formed in the reaction.(l) Solid ammonium carbonate is heated.Classify the type of reaction occurring.(m) A solution of hydrogen peroxide is catalyticallydecomposed.Name the element being reduced and the element beingoxidized.(n) A solution of potassium iodide is electrolyzed.At which electrode would a gas be released?(o) A solution of copper(II) sulfate is electrolyzed usinginert electrodes.Is the final solution acidic, basic or neutral?(p) A solution of ammonium sulfate is added to a saturatedsolution of barium hydroxide.Identify any precipitate formed in the reaction.(q) A solution of copper(II) chloride is added to a solutionof sodium sulfide.Name the spectator ions in this reaction.(r) Solutions of manganese(II) sulfate and ammoniumsulfide are mixed.List any precipitate that forms during the reaction.– 2 –Publication No. <strong>10826</strong>Your Safer Source for Science Supplies© 2007 Flinn Scientific, Inc. All Rights Reserved.(800) 452-1261 • Fax: (866) 452-1436 • P.O. Box 219 • Batavia, IL 60510 • E-mail: flinn@flinnsci.com • www.flinnsci.com
(s) Solutions of silver nitrate and sodium chromate aremixed.What is the oxidation number of chromium in the chromateion?(t) Glacial acetic acid is mixed with liquid methanol(nonaqueous).What type of organic reaction can this becharacterized as?(u) Chlorine gas is bubbled into a cold, dilute solution ofpotassium hydroxide.What element is undergoing oxidation and what elementis undergoing reduction?(v) A strip of copper is immersed in a concentratednitric acid solution.List at least two observations that indicate a chemicalreaction is occurring.(w) Hydrogen gas is passed over hot iron(II) oxidepowder.What is the oxidation number of the hydrogen in hydrogengas?(x) Acidified potassium permanganate is added to asolution of sodium nitrite.Write and balance the oxidation half-reaction for massand charge.(y) A solution of sodium bromide is added to an acidifiedsolution of potassium bromate.Write and balance the reduction half-reaction for massand charge.(z) Aluminum metal is added to a solution of copper(II)chloride.List at least two observations that indicate a chemicalreaction is occurring.(aa) Excess chlorine gas is passed over hot iron filings.What type of reaction is occurring?(bb) Magnesium metal is added to nitrogen gas.What is the oxidation number of magnesium before andafter the reaction?– 3 –Publication No. <strong>10826</strong>Your Safer Source for Science Supplies© 2007 Flinn Scientific, Inc. All Rights Reserved.(800) 452-1261 • Fax: (866) 452-1436 • P.O. Box 219 • Batavia, IL 60510 • E-mail: flinn@flinnsci.com • www.flinnsci.com
(cc) Solid lithium hydride is added to distilled water.Is the final solution acidic, basic or neutral? Explain.(dd) Benzene is treated with bromine in the presence of acatalyst.Classify the type of organic reaction that takes place.(ee) Solid lithium oxide is added to excess water.Is the final solution acidic, basic or neutral? Explain.(ff) Solid potassium chlorate is heated in the presence ofmanganese dioxide as a catalyst.How many moles of reaction products can be producedfrom one mole of potassium chlorate?(gg) Dilute hydrochloric acid is added to a solution ofpotassium sulfite.List all spectator ions.(hh) A solution of sulfuric acid is added to a solution ofbarium hydroxide until the same number of moles ofeach compound has been added.Is the final solution acidic, basic or neutral? Explain..(ii) A mixture of solid calcium oxide and solid tetraphosphorusdecaoxide is heated.Is the product compound(s) soluble in water?(jj) Sulfur dioxide gas is passed over solid calciumoxide.Name the product compound(s).(kk) Solid zinc sulfide is heated in an excess of oxygen.What change in oxidation state does sulfur undergo in thisreaction?(ll) A solution of potassium iodide is added to an acidifiedsolution of potassium dichromate.What is the reducing agent in this reaction?– 4 –Publication No. <strong>10826</strong>Your Safer Source for Science Supplies© 2007 Flinn Scientific, Inc. All Rights Reserved.(800) 452-1261 • Fax: (866) 452-1436 • P.O. Box 219 • Batavia, IL 60510 • E-mail: flinn@flinnsci.com • www.flinnsci.com
<strong>AP</strong> <strong>Chemistry</strong> <strong>Chemical</strong> <strong>Equations</strong> <strong>Worksheet</strong>Answer KeyChanges to the <strong>AP</strong> <strong>Chemistry</strong> Exam format for 2007 include modification to Question 4 in Section II. Previously, studentswere asked to write chemical equations for any five of eight given sets of chemical reactions. The new format requires studentsto write balanced chemical equations showing only the reacting substances (excluding spectator ions) for three different setsof reactants and to answer a short question (requiring no calculator) about each reaction. The questions are intended to test thestudents’ understanding of the meaning of the reactions.The College Board has provided only a few examples of the types of questions that might be asked. (Visit the CollegeBoard Web site at www.apcentral.collegeboard.com/apc/public/courses/150180.html?type=print for examples.) <strong>AP</strong> <strong>Chemistry</strong>teachers have asked us if we have any products or worksheets to address this new format. The following list of practicechemical equations is provided as a helpful tool to assist teachers. We have compiled these questions based on our interpretationof the questions on the College Board Web site. Flinn Scientific has no additional information or guidance other than thatprovided by the College Board on its Web site.In each of the 38 reactions listed below, a representative question about the reaction and the answer to the question areincluded, along with the balanced chemical equation. We hope that you will find this worksheet helpful in preparing studentsfor the new <strong>AP</strong> <strong>Chemistry</strong> chemical equation section.(a) A solution of ammonia is added to a dilutesolution of acetic acid.NH 3+ CH 3COOH → NH+4+ CH 3COO –(b) Solutions of sodium hydroxide and acetic acid aremixed.OH – + CH 3COOH → H 2O + CH 3COO –(c) Hydrogen sulfide gas is bubbled through excesspotassium hydroxide solution.H 2S + 2OH – → S 2– + 2H 2O(d) Solid barium oxide is added to distilled water.BaO + H 2O → Ba 2+ + 2OH –(e) Solid calcium oxide is exposed to a stream ofcarbon dioxide gas.Identify the conjugate acid–base pairs in this reaction.Answer: NH 3(base) and NH+4(acid)CH 3COOH (acid) and CH 3COO – (base)Acetic acid is a weak acid. If equal volumes of equalmolar solutions are mixed, will the solution pH be >7, 7,or 7. The salt of a weak acid (CH 3COO – ) isbasic in solution.Write the successive ionization equations for H 2S.Answer: H 2S + H 2O ← → HS – + H 3O +HS – + H 2O ← → S 2– + H 3O +Is the resulting solution acidic, basic or neutral? Explain.Answer: Basic. Metal oxides form basic compounds inwater.What type of reaction has occurred?Answer: A synthesis (or combination) reaction.CaO + CO 2→ CaCO 3(f) Solid dinitrogen pentoxide is added to water.N 2O 5+ H 2O → 2H + + 2NO–3(g) Carbon disulfide vapor is burned in excess oxygen.CS 2+ 3O 2→ CO 2+ 2SO 2Is the final solution acidic, basic or neutral? Explain.Answer: Acidic. Nonmetal oxides form acidic compoundsin water.What is the oxidizing agent in this reaction?Answer: Oxygen.– 5 –Publication No. <strong>10826</strong>Your Safer Source for Science Supplies© 2007 Flinn Scientific, Inc. All Rights Reserved.(800) 452-1261 • Fax: (866) 452-1436 • P.O. Box 219 • Batavia, IL 60510 • E-mail: flinn@flinnsci.com • www.flinnsci.com
(s) Solutions of silver nitrate and sodium chromate aremixed.2Ag + + CrO2–4→ Ag 2CrO 4(t) Glacial acetic acid is mixed with liquid methanol(nonaqueous).CH 3COOH + CH 3OH → CH 3COOCH 3+ H 2O(u) Chlorine gas is bubbled into a cold, dilute solution ofpotassium hydroxide.Cl 2+ 2OH – → ClO – + Cl – + H 2O(v) A strip of copper is immersed in a concentratednitric acid solution.Cu + 4H + + 2NO–3→ Cu 2+ + 2NO 2+ 2H 2O(w) Hydrogen gas is passed over hot iron(II) oxidepowder.H 2+ FeO → Fe + H 2O(x) Acidified potassium permanganate is added to asolution of sodium nitrite.2MnO–4+ 6H + + 5NO–2→2Mn 2+ + 3H 2O + 5NO–3(y) A solution of sodium bromide is added to an acidifiedsolution of potassium bromate.5Br – + 6H + + BrO–3→ 3Br 2+ 3H 2O(z) Aluminum metal is added to a solution of copper(II)chloride.2Al + 3Cu 2+ → 2Al 3+ + 3Cu(aa) Excess chlorine gas is passed over hot iron filings.3Cl 2+ 2Fe → 2FeCl 3(bb) Magnesium metal is added to nitrogen gas.3Mg + N 2→ Mg 3N 2What is the oxidation number of chromium in the chromateion?Answer: +6.What type of organic reaction can this becharacterized as?Answer: An esterification reaction.What element is undergoing oxidation and what elementis undergoing reduction?Answer: Chlorine, Cl, is being reduced to –1 (Cl – ) andalso being oxidized to +1 in ClO – .List at least two observations that indicate a chemicalreaction is occurring.Answer: Solution color changes from colorless to blue.Bubbles of NO 2gas are observed at the copperstrip. The gas is brown.What is the oxidation number of the hydrogen in hydrogengas?Answer: Zero—the oxidation number of an atom in itselemental form is zero.Write and balance the oxidation half-reaction for massand charge.Answer: H 2O + NO–2→ NO–3+ 2H + + 2e –Write and balance the reduction half-reaction for massand charge.Answer: 12H + + 2BrO–3+ 10e – → Br 2+ 6H 2OList at least two observations that indicate a chemicalreaction is occurring.Answer: The aluminum dissolves, a red solid precipitates,the blue color of the solution fades, andthe solution temperature increases.What type of reaction is occurring?Answer: A synthesis reaction (or a redox reaction).What is the oxidation number of magnesium before andafter the reaction?Answer: Magnesium goes from zero in Mg to +2 inMg 3N 2.– 7 –Publication No. <strong>10826</strong>Your Safer Source for Science Supplies© 2007 Flinn Scientific, Inc. All Rights Reserved.(800) 452-1261 • Fax: (866) 452-1436 • P.O. Box 219 • Batavia, IL 60510 • E-mail: flinn@flinnsci.com • www.flinnsci.com
(cc) Solid lithium hydride is added to distilled water.LiH + H 2O → Li + + OH – + H 2(dd) Benzene is treated with bromine in the presence of acatalyst.Br 2+ C 6H 6→ C 6H 5Br + HBr(ee) Solid lithium oxide is added to excess water.Li 2O + H 2O → 2Li + + 2OH –(ff) Solid potassium chlorate is heated in the presence ofmanganese dioxide as a catalyst.MnO2KClO 23⎯⎯→ 2KCl + 3O 2(gg) Dilute hydrochloric acid is added to a solution ofpotassium sulfite.Is the final solution acidic, basic or neutral? Explain.Answer: Basic. Metallic hydrides react with water toform metallic hydroxides.Classify the type of organic reaction that takes place.Answer: This is a substitution reaction.Is the final solution acidic, basic or neutral? Explain.Answer: Basic. Soluble metal oxides react in water toform bases (metallic hydroxides).How many moles of reaction products can be producedfrom one mole of potassium chlorate?Answer: 2½ moles.List all spectator ions.Answer: Potassium, K + , and chloride, Cl – .2H + + SO 32–→ H 2O + SO 2(hh) A solution of sulfuric acid is added to a solution ofbarium hydroxide until the same number of moles ofeach compound has been added.2H + + SO2–4+ Ba 2+ + 2OH – → BaSO 4+ 2H 2O(ii) A mixture of solid calcium oxide and solid tetraphosphorusdecaoxide is heated.6CaO + P 4O 10→ 2Ca 3(PO 4) 2(jj) Sulfur dioxide gas is passed over solid calciumoxide.Is the final solution acidic, basic or neutral? Explain.Answer: Neutral. The neutralization of a strong base[Ba(OH) 2] by a strong acid [H 2SO 4] yields aneutral solution.Is the product compound(s) soluble in water?Answer: No. Phosphates other than those of group 1elements and NH+4are insoluble.Name the product compound(s).Answer: Calcium sulfite.SO 2+ CaO → CaSO 3(kk) Solid zinc sulfide is heated in an excess of oxygen.2ZnS + 3O 2→ 2ZnO + 2SO 2(ll) A solution of potassium iodide is added to an acidifiedsolution of potassium dichromate.6I – + 14H + + Cr 2O2–7→ 3I 2+ 2Cr 3+ + 7H 2OWhat change in oxidation state does sulfur undergo in thisreaction?Answer: Sulfur starts out as –2 in ZnS and ends up as+4 in SO 2. The charge is +6.What is the reducing agent in this reaction?Answer: The species that undergoes oxidation, in thiscase iodide ion, I – , is the reducing agent.– 8 –Publication No. <strong>10826</strong>Your Safer Source for Science Supplies© 2007 Flinn Scientific, Inc. All Rights Reserved.(800) 452-1261 • Fax: (866) 452-1436 • P.O. Box 219 • Batavia, IL 60510 • E-mail: flinn@flinnsci.com • www.flinnsci.com