Packed Bed flooding.pdf - Youngstown State University's Personal ...
Packed Bed flooding.pdf - Youngstown State University's Personal ... Packed Bed flooding.pdf - Youngstown State University's Personal ...
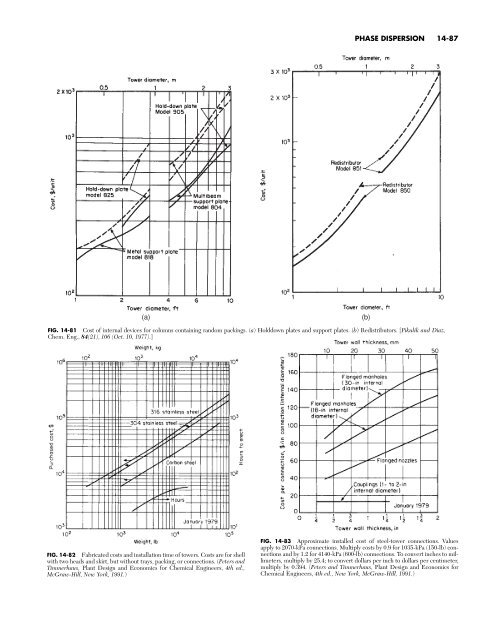
(a) PHASE DISPERSION 14-87 FIG. 14-81 Cost of internal devices for columns containing random packings. (a) Holddown plates and support plates. (b) Redistributors. [Pikulik and Diaz, Chem. Eng., 84(21), 106 (Oct. 10, 1977).] FIG. 14-82 Fabricated costs and installation time of towers. Costs are for shell with two heads and skirt, but without trays, packing, or connections. (Peters and Timmerhaus, Plant Design and Economics for Chemical Engineers, 4th ed., McGraw-Hill, New York, 1991.) FIG. 14-83 Approximate installed cost of steel-tower connections. Values apply to 2070-kPa connections. Multiply costs by 0.9 for 1035-kPa (150-lb) connections and by 1.2 for 4140-kPa (600-lb) connections. To convert inches to millimeters, multiply by 25.4; to convert dollars per inch to dollars per centimeter, multiply by 0.394. (Peters and Timmerhaus, Plant Design and Economics for Chemical Engineers, 4th ed., New York, McGraw-Hill, 1991.) (b)
14-88 EQUIPMENT FOR DISTILLATION, GAS ABSORPTION, PHASE DISPERSION, AND PHASE SEPARATION FIG. 14-84 Cost of towers including installation and auxiliaries. To convert inches to millimeters, multiply by 25.4; to convert feet to meters, multiply by 0.305; and to convert dollars per foot to dollars per meter, multiply by 3.28. (Peters and Timmerhaus, Plant Design and Economics for Chemical Engineers, 4th ed., McGraw-Hill, New York, 1991.) However, droplet systems can enable much higher energy input (via gas phase pressure drop in cocurrent systems) and, as a result, dominate applications where a quick quench is needed. See Examples 21 and 22. Conversely, droplet systems can also be designed for very low pressure drop which is advantageous in applications such as vacuum condensers. Unstable Systems: Froths and Hollow Cone Atomizing Nozzles We usually think of interfacial contact as a steady-state system of raining droplets or rising bubbles, but some of the most efficient interfacial contactors take advantage of unstable interfacial geometry. The most common is the distillation tray which operates with a wild mix of bubbles, jets, films, and droplets. The mix is often described as froth. Gas pressure drop provides the energy to create the froth. A variant on the froth contact is the reverse jet contactor (Example 22), which can be considered as an upside-down distillation tray, operated above the flooding velocity in cocurrent flow of gas and liquid. It is limited to one stage. An entirely different unstable contactor involves the thin expanding liquid film produced by a hollow cone spray nozzle. Because of fresh surface and the thinness of the film, this can give very high transfer for liquid-limited systems. Two applications are direct contact condensation and removal of volatile components from a high-boiling residual liquid. Surface Tension Makes Liquid Sheets and Liquid Columns Unstable Surface tension is the energy required to make an increment of interfacial surface. A sheet or column of liquid has more surface than a sphere, hence surface tension converts sheets and columns to droplets. See Fig. 14-86. There are many different atomizers, but the underlying principle of all is the same—to first generate a flat sheet or a liquid column. Liquid sheets and columns are unstable, a small surface disturbance on either will propagate, and the liquid will reshape itself into droplets. The key property in controlling this process is surface tension. Surface tension gets a high exponent in all the atomization correlations. Little Droplets and Bubbles vs. Big Droplets and Bubbles— Coalescence vs. Breakup When big drops are subjected to shear forces, as in falling rain, the droplets are distorted; and if the distor- tions are great enough, the big droplets break into little ones. This is why raindrops never exceed a certain size. A variant on this is breakup in highly turbulent systems such as in high-velocity quench systems or pneumatic nozzles. Here the droplets are distorted by the energy of the turbulent eddies. But little droplets and bubbles have more surface per unit of liquid than big ones. Hence little droplets tend to coalesce into big ones, and will grow in size if given enough quiet time. While the primary difficulty is estimating the interfacial area due to the unstable interface, a secondary problem is that freshly made, unstable surface gives higher transfer than older, more stable surface. Empirical Design Tempered by Operating Data The net of these is that interfacial area is difficult to predict and interfacial contactors are difficult to design. Prediction methods are given below but should always be tempered by operating experience. INTERFACIAL AREA—IMPACT OF DROPLET OR BUBBLE SIZE Transfer is aided by increased interfacial area. Interfacial area per unit volume aD of a single droplet or bubble is inversely proportional to the diameter of the droplet or bubble D. aD = 6/D (14-177) To estimate the total interfacial area in a given volume, the ad value is multiplied by the fractional holdup of dispersed phase in the total volume. a = aD(ΦD) (14-178) where a = interfacial area/volume and ΦD = fraction of volume in dispersed phase = holdup. Fractional holdup in a continuous process depends on the velocities of the two phases, as if they were flowing by themselves. ΦD = (dispersed phase volume)/(volume of dispersed and continuous phases) Example 14: Interfacial Area for Droplets/Gas in Cocurrent Flow For equal mass flow of gas and liquid and with gas density 0.001 of liquid density, the gas velocity in cocurrent flow will be 1000 times the liquid velocity. This sets Φ D. ΦD = 1/(1 + 1000) = 0.00099 If the droplets are 500 µm in diameter, Eqs. (14-177) and (14-178) give a = (6/0.0005)(0.00099) = 12 m2 /m3 If the droplets are 100 µm in diameter, Eqs. (14-177) and (14-178) give a = (6/0.0001)(0.00099) = 60 m2 /m3 Example 15: Interfacial Area for Droplets Falling in a Vessel Droplet systems rarely exceed a ΦD value of 0.01. At this low level, ΦD in a lowvelocity countercurrent contactor can be approximated by Eq. (14-179). ΦD = UL/(Ut − UG) (14-179) where UL = liquid superficial velocity Ut = terminal velocity of droplet UG = gas superficial velocity With a gas superficial velocity of 1.5 m/s, for equal mass flow of gas and liquid, with gas density 0.001 of liquid density, and with 500-µm-diameter droplets falling at a terminal settling of 2.5 m/s, Eq. (14-179) gives a fractional holdup of liquid of ΦD = (0.001)1.5/(2.5 − 1.5) = 0.0015 Equations (14-177) and (14-178) then give a = (6/0.0005)(0.0015) = 18 m 2 /m 3 Example 16: Interfacial Area for Bubbles Rising in a Vessel For bubble systems (gases dispersed in liquids) fractional holdup can approach 0.5 as shown by Fig. 14-104. However, before reaching this holdup, the bubble systems shift to an unstable mix of bubbles and vapor jets. Hence an exact
- Page 1 and 2: Previous Page 14-58 EQUIPMENT FOR D
- Page 3 and 4: 14-60 TABLE 14-13 Characteristics o
- Page 5 and 6: 14-62 EQUIPMENT FOR DISTILLATION, G
- Page 7 and 8: 14-64 EQUIPMENT FOR DISTILLATION, G
- Page 9 and 10: 14-66 EQUIPMENT FOR DISTILLATION, G
- Page 11 and 12: 14-68 EQUIPMENT FOR DISTILLATION, G
- Page 13 and 14: 14-70 EQUIPMENT FOR DISTILLATION, G
- Page 15 and 16: 14-72 EQUIPMENT FOR DISTILLATION, G
- Page 17 and 18: 14-74 EQUIPMENT FOR DISTILLATION, G
- Page 19 and 20: 14-76 EQUIPMENT FOR DISTILLATION, G
- Page 21 and 22: 14-78 (a) (b) (c) FIG. 14-71 High-v
- Page 23 and 24: 14-80 EQUIPMENT FOR DISTILLATION, G
- Page 25 and 26: 14-82 EQUIPMENT FOR DISTILLATION, G
- Page 27 and 28: 14-84 EQUIPMENT FOR DISTILLATION, G
- Page 29: 14-86 EQUIPMENT FOR DISTILLATION, G
- Page 33 and 34: 14-90 EQUIPMENT FOR DISTILLATION, G
- Page 35 and 36: 14-92 EQUIPMENT FOR DISTILLATION, G
- Page 37 and 38: 14-94 EQUIPMENT FOR DISTILLATION, G
- Page 39 and 40: 14-96 EQUIPMENT FOR DISTILLATION, G
- Page 41 and 42: 14-98 EQUIPMENT FOR DISTILLATION, G
- Page 43 and 44: 14-100 EQUIPMENT FOR DISTILLATION,
- Page 45 and 46: 14-102 EQUIPMENT FOR DISTILLATION,
- Page 47 and 48: 14-104 EQUIPMENT FOR DISTILLATION,
- Page 49 and 50: 14-106 EQUIPMENT FOR DISTILLATION,
- Page 51 and 52: 14-108 EQUIPMENT FOR DISTILLATION,
- Page 53 and 54: 14-110 EQUIPMENT FOR DISTILLATION,
- Page 55 and 56: 14-112 EQUIPMENT FOR DISTILLATION,
- Page 57 and 58: 14-114 EQUIPMENT FOR DISTILLATION,
- Page 59 and 60: 14-116 EQUIPMENT FOR DISTILLATION,
- Page 61 and 62: 14-118 EQUIPMENT FOR DISTILLATION,
- Page 63 and 64: 14-120 EQUIPMENT FOR DISTILLATION,
- Page 65 and 66: 14-122 EQUIPMENT FOR DISTILLATION,
- Page 67 and 68: 14-124 EQUIPMENT FOR DISTILLATION,
- Page 69 and 70: 14-126 EQUIPMENT FOR DISTILLATION,
- Page 71 and 72: 14-128 EQUIPMENT FOR DISTILLATION,
(a)<br />
PHASE DISPERSION 14-87<br />
FIG. 14-81 Cost of internal devices for columns containing random packings. (a) Holddown plates and support plates. (b) Redistributors. [Pikulik and Diaz,<br />
Chem. Eng., 84(21), 106 (Oct. 10, 1977).]<br />
FIG. 14-82 Fabricated costs and installation time of towers. Costs are for shell<br />
with two heads and skirt, but without trays, packing, or connections. (Peters and<br />
Timmerhaus, Plant Design and Economics for Chemical Engineers, 4th ed.,<br />
McGraw-Hill, New York, 1991.)<br />
FIG. 14-83 Approximate installed cost of steel-tower connections. Values<br />
apply to 2070-kPa connections. Multiply costs by 0.9 for 1035-kPa (150-lb) connections<br />
and by 1.2 for 4140-kPa (600-lb) connections. To convert inches to millimeters,<br />
multiply by 25.4; to convert dollars per inch to dollars per centimeter,<br />
multiply by 0.394. (Peters and Timmerhaus, Plant Design and Economics for<br />
Chemical Engineers, 4th ed., New York, McGraw-Hill, 1991.)<br />
(b)