Download - Lohmann Animal Health
Download - Lohmann Animal Health Download - Lohmann Animal Health
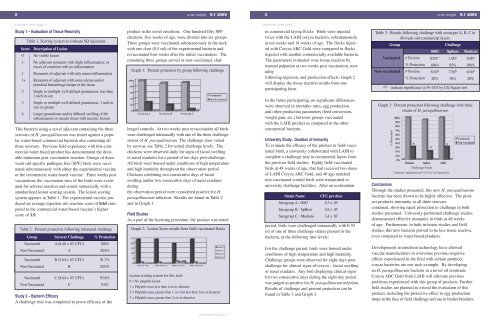
1240.AvianInsight Vol1-05 1/10/05 3:13 PM Page 22 avian insightV.1 20053 avian insightV.1 2005continued from page 1continued from page 2Study 1 – Evaluation of Tissue ReactivityScoreTable 1. Scoring system to evaluate SQ injectionsDescription of Lesion0 No visible lesion1- No adjuvant remnants with slight inflammation; ortraces of emulsion with no inflammation1 Remnants of adjuvant with only minor inflammation1+ Remnants of adjuvant with some edema and/orpetechial hemorrhage deeper in the tissue2 Single or multiple well-defined granulomas, less then1 inch in size3 Single or multiple well-defined granulomas, 1 inch insize or greater4 Larger granulomas and/or diffused swelling of thesubcutaneous or muscle tissue with necrotic lesionsThis bacterin using a novel adjuvant containing the threeserovars of H. paragallinarum was tested against a popularwater-based commercial bacterin also containing allthree serovars. Previous field experience with this commercialwater-based product has demonstrated the desirableminimum post vaccination reaction. Groups of threeweek-oldspecific pathogen free (SPF) birds were vaccinatedsubcutaneously with either the experimental vaccineor the commercial water-based vaccine. Three weeks postvaccination, the vaccination sites of the birds were evaluatedfor adverse reaction and scored numerically with astandardized lesion scoring system. The lesion scoringsystem appears in Table 1. The experimental vaccine producedan average injection site reaction score of 0.63 comparedto the commercial water-based vaccine’s higherscore of 1.9.Table 2. Percent protection following intranasal challengeGroup Serovar Challenge % ProtectionGraph 1: Percent protection by group following challengeproduct in the novel emulsion. One hundred fifty SPFchickens, five weeks of age, were divided into six groups.Three groups were vaccinated subcutaneously in the neckwith one dose (0.5 ml) of the experimental bacterin andrevaccinated four weeks after the initial vaccination. Theremaining three groups served as non-vaccinated, challengedcontrols. At two weeks post revaccination all birdswere challenged intranasally with one of the three challengestrains of H. paragallinarum. The challenge dose variedby serovar, see Table 2 for actual challenge levels. Thechickens were observed daily for signs of facial swellingor nasal exudates for a period of ten days post-challenge.All birds were housed under conditions of high temperatureand high humidity throughout the observation period.Chickens exhibiting two consecutive days of facialswelling and/or two consecutive days of nasal exudatesduringthe observation period were considered positive for H.paragallinarum infection. Results are found in Table 2and in Graph 1.Field StudiesAs a part of the licensing procedure, the product was testedGraph 2: Lesion Score results from field-vaccinated flocksin commercial laying flocks. Birds were injectedtwice with the LAHI coryza bacterin, subcutaneouslyat ten weeks and 14 weeks of age. The flocks injectedwith Coryza ABC Gold were compared to flocksinjected with another commercially available bacterin.The parameters evaluated were tissue reaction bymanual palpation at two weeks post vaccination, mortalityfollowing injection, and production effects. Graph 2will display the tissue reaction results from oneparticipating farm.In the farms participating, no significant differenceswere observed in mortality rates, egg productionand other production parameters (feed conversion,weight gain, etc.) between groups vaccinatedwith the LAHI product as compared to the othercommercial bacterin.University Study: Duration of ImmunityTo evaluate the efficacy of the product in field-vaccinatedbirds, a university collaborated with LAHI tocomplete a challenge trial in commercial layers fromthe previous field studies. Eighty field-vaccinatedbirds at 48 weeks of age, that had received two dosesof LAHI Coryza ABC Gold, and 40 age-matchednon-vaccinated control birds were transported touniversity challenge facilities. After an acclimationStrain NameCFU per doseSerogroup A - 0083 8.3 x 10 6Serogroup B - SpRoss 6.8 x 10 5Serogroup C - Modesto 3.4 x 10 7period, birds were challenged intranasally with 0.30ml of one of three challenge strains present in thebacterin, at the following titer levels:Table 3: Results following challenge with serotypes A, B, C in48-week-old commercial layers.GroupVaccinatedNon-vaccinated# Positive% Protection# Positive% ProtectionChallenge0083 SpRoss Modesto0/20 a--100%8/10 b20%1/20 a95%7/10 b30%a,b - indicate significance at P< 0.01 by Chi Square test0/20 a100%8/10 b20%Graph 3: Percent protection following challenge with threestrains of H. paragallinarum.ConclusionsThrough the studies presented, this new H. paragallinarumbacterin has been shown to be highly effective. The productproduces immunity to all three serovarscontained, showing equal protection to challenge in bothstudies presented. University-performed challenge studiesdemonstrated effective immunity in birds at 48 weeksof age. Furthermore, in both in-house studies and fieldstudies, this new bacterin proved to be less tissue reactive,even compared to water-based products.Vaccinated A (8.46 x 10 7 CFU) 100%Non-Vaccinated A 28.6%Vaccinated B (5.64 x 10 5 CFU) 91.7%Non-Vaccinated B 20.0%Vaccinated C (8.64 x 10 7 CFU) 93.8%Non-Vaccinated C 5.6%Study 2 – Bacterin EfficacyA challenge trial was completed to prove efficacy of theLesion scoring system for this trial:0 = No palpable lesion1 = Palpable mass less than 1cm in diameter2 = Palpable mass greater than 1 cm, but less than 2cm in diameter3 = Palpable mass greater than 2 cm in diameterFor the challenge period, birds were housed underconditions of high temperature and high humidity.Challenge groups were observed for eight days postchallengefor clinical signs of coryza – facial swellingor nasal exudates. Any bird displaying clinical signsfor two consecutive days during the eight-day periodwas judged as positive for H. paragallinarum infection.Results of challenge and percent protection can befound in Table 3 and Graph 3.Developments in emulsion technology have allowedvaccine manufacturers to overcome previous negativeeffects experienced in the field with certain products;coryza bacterins are one such example. By developingan H. paragallinarum bacterin in a novel oil emulsion,Coryza ABC Gold from LAHI will alleviate previousproblems experienced with this group of products. Furtherfield studies are planned to extend the evaluation of thisproduct, including the protective effect to egg productiondrops in the face of field challenge and use in broiler breeders.continued on page 3
1240.AvianInsight Vol1-05 1/10/05 3:13 PM Page 22 avian insightV.1 20053 avian insightV.1 2005continued from page 1continued from page 2Study 1 – Evaluation of Tissue ReactivityScoreTable 1. Scoring system to evaluate SQ injectionsDescription of Lesion0 No visible lesion1- No adjuvant remnants with slight inflammation; ortraces of emulsion with no inflammation1 Remnants of adjuvant with only minor inflammation1+ Remnants of adjuvant with some edema and/orpetechial hemorrhage deeper in the tissue2 Single or multiple well-defined granulomas, less then1 inch in size3 Single or multiple well-defined granulomas, 1 inch insize or greater4 Larger granulomas and/or diffused swelling of thesubcutaneous or muscle tissue with necrotic lesionsThis bacterin using a novel adjuvant containing the threeserovars of H. paragallinarum was tested against a popularwater-based commercial bacterin also containing allthree serovars. Previous field experience with this commercialwater-based product has demonstrated the desirableminimum post vaccination reaction. Groups of threeweek-oldspecific pathogen free (SPF) birds were vaccinatedsubcutaneously with either the experimental vaccineor the commercial water-based vaccine. Three weeks postvaccination, the vaccination sites of the birds were evaluatedfor adverse reaction and scored numerically with astandardized lesion scoring system. The lesion scoringsystem appears in Table 1. The experimental vaccine producedan average injection site reaction score of 0.63 comparedto the commercial water-based vaccine’s higherscore of 1.9.Table 2. Percent protection following intranasal challengeGroup Serovar Challenge % ProtectionGraph 1: Percent protection by group following challengeproduct in the novel emulsion. One hundred fifty SPFchickens, five weeks of age, were divided into six groups.Three groups were vaccinated subcutaneously in the neckwith one dose (0.5 ml) of the experimental bacterin andrevaccinated four weeks after the initial vaccination. Theremaining three groups served as non-vaccinated, challengedcontrols. At two weeks post revaccination all birdswere challenged intranasally with one of the three challengestrains of H. paragallinarum. The challenge dose variedby serovar, see Table 2 for actual challenge levels. Thechickens were observed daily for signs of facial swellingor nasal exudates for a period of ten days post-challenge.All birds were housed under conditions of high temperatureand high humidity throughout the observation period.Chickens exhibiting two consecutive days of facialswelling and/or two consecutive days of nasal exudatesduringthe observation period were considered positive for H.paragallinarum infection. Results are found in Table 2and in Graph 1.Field StudiesAs a part of the licensing procedure, the product was testedGraph 2: Lesion Score results from field-vaccinated flocksin commercial laying flocks. Birds were injectedtwice with the LAHI coryza bacterin, subcutaneouslyat ten weeks and 14 weeks of age. The flocks injectedwith Coryza ABC Gold were compared to flocksinjected with another commercially available bacterin.The parameters evaluated were tissue reaction bymanual palpation at two weeks post vaccination, mortalityfollowing injection, and production effects. Graph 2will display the tissue reaction results from oneparticipating farm.In the farms participating, no significant differenceswere observed in mortality rates, egg productionand other production parameters (feed conversion,weight gain, etc.) between groups vaccinatedwith the LAHI product as compared to the othercommercial bacterin.University Study: Duration of ImmunityTo evaluate the efficacy of the product in field-vaccinatedbirds, a university collaborated with LAHI tocomplete a challenge trial in commercial layers fromthe previous field studies. Eighty field-vaccinatedbirds at 48 weeks of age, that had received two dosesof LAHI Coryza ABC Gold, and 40 age-matchednon-vaccinated control birds were transported touniversity challenge facilities. After an acclimationStrain NameCFU per doseSerogroup A - 0083 8.3 x 10 6Serogroup B - SpRoss 6.8 x 10 5Serogroup C - Modesto 3.4 x 10 7period, birds were challenged intranasally with 0.30ml of one of three challenge strains present in thebacterin, at the following titer levels:Table 3: Results following challenge with serotypes A, B, C in48-week-old commercial layers.GroupVaccinatedNon-vaccinated# Positive% Protection# Positive% ProtectionChallenge0083 SpRoss Modesto0/20 a--100%8/10 b20%1/20 a95%7/10 b30%a,b - indicate significance at P< 0.01 by Chi Square test0/20 a100%8/10 b20%Graph 3: Percent protection following challenge with threestrains of H. paragallinarum.ConclusionsThrough the studies presented, this new H. paragallinarumbacterin has been shown to be highly effective. The productproduces immunity to all three serovarscontained, showing equal protection to challenge in bothstudies presented. University-performed challenge studiesdemonstrated effective immunity in birds at 48 weeksof age. Furthermore, in both in-house studies and fieldstudies, this new bacterin proved to be less tissue reactive,even compared to water-based products.Vaccinated A (8.46 x 10 7 CFU) 100%Non-Vaccinated A 28.6%Vaccinated B (5.64 x 10 5 CFU) 91.7%Non-Vaccinated B 20.0%Vaccinated C (8.64 x 10 7 CFU) 93.8%Non-Vaccinated C 5.6%Study 2 – Bacterin EfficacyA challenge trial was completed to prove efficacy of theLesion scoring system for this trial:0 = No palpable lesion1 = Palpable mass less than 1cm in diameter2 = Palpable mass greater than 1 cm, but less than 2cm in diameter3 = Palpable mass greater than 2 cm in diameterFor the challenge period, birds were housed underconditions of high temperature and high humidity.Challenge groups were observed for eight days postchallengefor clinical signs of coryza – facial swellingor nasal exudates. Any bird displaying clinical signsfor two consecutive days during the eight-day periodwas judged as positive for H. paragallinarum infection.Results of challenge and percent protection can befound in Table 3 and Graph 3.Developments in emulsion technology have allowedvaccine manufacturers to overcome previous negativeeffects experienced in the field with certain products;coryza bacterins are one such example. By developingan H. paragallinarum bacterin in a novel oil emulsion,Coryza ABC Gold from LAHI will alleviate previousproblems experienced with this group of products. Furtherfield studies are planned to extend the evaluation of thisproduct, including the protective effect to egg productiondrops in the face of field challenge and use in broiler breeders.continued on page 3