Chem 232 Problem Set 3 Key
Chem 232 Problem Set 3 Key
Chem 232 Problem Set 3 Key
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
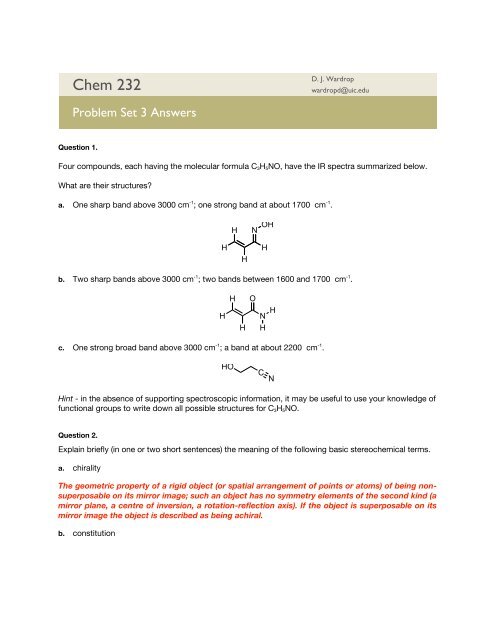
<strong>Chem</strong> <strong>232</strong>D. J. Wardropwardropd@uic.edu<strong>Problem</strong> <strong>Set</strong> 3 AnswersQuestion 1.Four compounds, each having the molecular formula C 3 H 5 NO, have the IR spectra summarized below.What are their structures?a. One sharp band above 3000 cm -1 ; one strong band at about 1700 cm -1 .HNOHHHHb. Two sharp bands above 3000 cm -1 ; two bands between 1600 and 1700 cm -1 .HHHONHHc. One strong broad band above 3000 cm -1 ; a band at about 2200 cm -1 .HOCNHint - in the absence of supporting spectroscopic information, it may be useful to use your knowledge offunctional groups to write down all possible structures for C 3 H 5 NO.Question 2.Explain briefly (in one or two short sentences) the meaning of the following basic stereochemical terms.a. chiralityThe geometric property of a rigid object (or spatial arrangement of points or atoms) of being nonsuperposableon its mirror image; such an object has no symmetry elements of the second kind (amirror plane, a centre of inversion, a rotation-reflection axis). If the object is superposable on itsmirror image the object is described as being achiral.b. constitution
The description of the identity and connectivity (and corresponding bond multiplicities) of theatoms in a molecular entity (omitting any distinction arising from their spatial arrangement).c. configurationIn the context of stereochemistry, the term is restricted to the arrangements of atoms of amolecular entity in space that distinguishes stereoisomers, the isomerism between which is notdue to conformation differences.d. conformationThe spatial arrangement of the atoms affording distinction between stereoisomers which can beinterconverted by rotations about formally single bonds. Some authorities extend the term toinclude inversion at trigonal pyramidal centres and other polytopal rearrangements.e. stereoisomersIsomers that possess identical constitution, but which differ in the arrangement of their atoms inspace.Question 3.Under what circumstances is a molecule or object chiral?An object is chiral when it lacks an internal plane of symmetry and has a non-superimposable mirrorimage.Question 4.What relationship do the following pairs of compounds have with each other?(a)(b)H 3 CO CH 3CH 3OandCH 3CHCH 33CHCH 3 3configurational isomers(enantiomers)CH 3 CH 3andH 3 CH 3 CConformational isomersO CH 3CH 3CH 3CH 3(c)(d)CH 3 CH 3andOHO OHandH 3 CConformational isomers (duh!)HHConstituitional isomersOOHHCH 3OH
Question 5.Which of the following molecules are chiral, which are achiral? If necessary, draw diagrams to justifyyour answers.(1R,3S)-cyclohexane-1,3-diol(1R,3R)-cyclohexane-1,3-diolHOOHAchiral (chiral centers,but plane of symmetry = meso)HOOHChiral (no plane of symmetry)OHOHOCO 2 HOHOHAchiral (chiral centers,but plane of symmetry = meso)OAchiral (no chiral centers= plane of symmetry)OOHO 2 C•CO 2 HAchiral (point of inversion •)CO 2 HHO 2 CBrChiral (no point of inversion •)Achiral (no chiral centers)HOOHOOHHO 2 CO OHClBrFOOHOHO 2 COHCO 2 HAchiral (no chiral centers= plane of symmetry)HOOHAchiral (chiral centers,but plane of symmetry = meso)Chiral (no chiral center)OHO O MeChiral (no plane of symmetry)Question 6.Draw the structure of all possible stereoisomers of 2-methylcyclohexan-1-ol.What relationship do these isomers have to one another?OHOH(1R,2S)-2-methylcyclohexanol(1R,2R)-2-methylcyclohexanolenantiomersenantiomersOHOH(1S,2R)-2-methylcyclohexanol(1S,2S)-2-methylcyclohexanoldiastereomers
!Question 7.Using the Cahn–Ingold–Prelog (CIP) priority rules, determine the absolute configuration and assign an Ror S descriptor to each chirality center within the following molecules.H 2 N HMeROHOOHR SH BrHOMeH MeRSHOOHO HSOHOHONHH 2 NCO 2 HHCH 2 SHHO 2 NHOCF 3R RMeRSOCHOOHRHCH 3HOHSOHBrRQuestion 8.Which of the following properties or methods can be used to distinguish between R-(-)-carvone and S-(+)-carvone?OOa. boiling pointb. refractive indexc. melting pointd. smelle. optical rotationf. dipole momentg. IR spectroscopyHR-(-)-carvoneHS-(+)-carvone
Question 9.Mark all the chirality centers in the structure of the lipid-lowering drug Lovastatin and the cytotoxicalkaloids Luotonin A and Ritterazine N with an asterisk (*). How many chirality centers are present ineach structure?8 Stereogenic (chiral) centersHO O0 Stereogenic (chiral) centersMeMeOOOMeNLuotonin ANNOMeOnot a chiralcenter!LovastatinOHOONNRitterazine NOOH20 Stereogenic (chiral) centersMaximum possible stereoisomers = 1,048,576OOnot a chiralcenter!
Question 10.For each of the following hydroboration-oxidation reactions, draw all of the possible stereoisomericproducts. Assume that the hydroboration step is regioselective.AchiralsubstrateAchiralreagent50 : 50(1R,2R)-2-methylcyclohexanol1. BH 3 •SMe 22. H 2 O 2 , NaOHOHOHRacemateChiralsubstrateAchiralreagent(1S,2S)-2-methylcyclohexanolH1. BH 3 •SMe 22. H 2 O 2 , NaOHHOHsingle isomer generatedthrough diastereo- andregioselectivehydroboration-oxidationRacemic Substrate50 : 50 mixture of both enantiomersH1. BH 3 •SMe 2HH1. BH 3 •SMe 2H2. H 2 O 2 , NaOHOHHO2. H 2 O 2 , NaOHAchiralreagentboth products formed throughdiastereoselectivehydroboration-oxidation
Question 11.For each of the following transformations, check the boxes which appropriate describe the observedstereo and regiochemical outcome.OOHn-PrOHH +On-PrONaHOOe.e. > 98.5 % e.e. > 98.5 %e.e. > 98.5 %XRegioselectiveXRegioselectiveEnantioselectiveEnantioselectiveXDiastereoselectiveXDiastereoselectiveXStereospecificXStereospecificOOPh1. BH 3 •THF2. H 2 O 2 , NaOHOOHOPhOOHOPh89% 11%XRegioselectiveEnantioselectiveXDiastereoselectiveStereospecificQuestion 12.The chromatographic purification of 1.0 g of ethyl R-(-)-lactate with an enantiomeric excess (e.e.) of 85%yields, without any loss of material, the enantiomeric pure (-)-enantiomer. How many grams of the (+)-enantiomer were separated?OOOHethyl (R)-(-)-lactatepurificationyield?OOOHethyl (R)-(-)-lactateee = 85%ee = 100%An enantiomeric excess of 85% corresponds to a 92.5/7.5 ratio of (-)-ethyl lactate to (+)-ethyl lactate. Inother words, 1 gram of "(-)-ethyl lactate with an enantiomeric excess (ee) of 85%" is a mixture of 925 mgof the (-)-enantiomer together with 75 mg of the (+)-enantiomer.