FMC Alubra brochure8 comp:Layout 1 - FMC BioPolymer
FMC Alubra brochure8 comp:Layout 1 - FMC BioPolymer
FMC Alubra brochure8 comp:Layout 1 - FMC BioPolymer
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
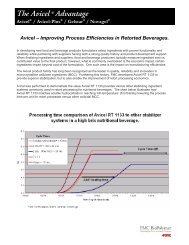
<strong>Alubra</strong> provides greater tablet strength.Trials were run on <strong>Alubra</strong> to evaluate its <strong>comp</strong>ressionperformance versus magnesium stearate. The results indicatethat <strong>Alubra</strong> offers superior <strong>comp</strong>ression versus magnesiumstearate across the <strong>comp</strong>lete <strong>comp</strong>ression profile. Furthermore,<strong>Alubra</strong> shows no drop-off in hardness versus magnesiumstearate at higher <strong>comp</strong>ression forces.Hardness (N)500450400350300250200Comparative Tablet HardnessAvicel ® PH-102 neatAvicel ® PH-102 + 1% <strong>Alubra</strong> Avicel ® PH-102 + 1% MgStWhen <strong>comp</strong>actability was measured on a rotary press, theblend using <strong>Alubra</strong> resulted in the hardest tablets, indicatingthat <strong>Alubra</strong> is a better choice than magnesium stearate whenit comes to high-speed tableting, and/or higher <strong>comp</strong>ressionforces.1501005005Source: <strong>FMC</strong> <strong>BioPolymer</strong> 201010 15 20 25 30 35Compression Force (kN)FORMULAIngredient%FormulaAvicel ® PH-102: 99<strong>Alubra</strong> or Magnesium Stearate: 1PROCEDURE• Materials: Avicel ® PH-102 + 1% lubricant(<strong>Alubra</strong> or MgSt) and Avicel ® PH-102 neat• Sieved through 710µm• Blending time 5 minutes• Rotary press: Manesty D4• One set of round, normal concave, D-type,12 mm diameter punches (position #1)• Tablet weight: 500 mg• Rotation speed: 34 RPM• Compression force: 5, 10, 15, 20, 25, 30 kN(for Avicel ® PH-102 neat: 2, 4, 8, 12, 16, 20 kN)tablet strength and quality.<strong>Alubra</strong> meets the highest quality standards.<strong>Alubra</strong> is manufactured under IPEC and cGMP conditions,which <strong>comp</strong>ly with recognized global quality standards. <strong>FMC</strong>’sown quality initiatives are designed to produce a lubricant thatis chemically, physically, and functionally consistent, as wellas <strong>comp</strong>liant with the stringent requirements of all majorpharmacopoeias. <strong>FMC</strong> carries out systematic product evaluationto ensure that <strong>Alubra</strong> continues to meet or surpass thesehigh standards.<strong>Alubra</strong> sodium stearyl fumarate is included in the FDAInactive Ingredient Database for oral capsules and tablets,as well as in the Canadian List of Acceptable Non-MedicinalIngredients.<strong>Alubra</strong> Commercial Information<strong>Alubra</strong> <strong>comp</strong>lies with all major pharmacopoeias:• NF • PH. Eur. • CP• JPE• BPCommercial packaging:• 1 KG (fiber)• 5 KG (fiber)• 20 KG (fiber)Available supporting documentation for <strong>Alubra</strong> :• Specification Sheet• MSDS• COAs• BSE/TSE Certificate• GMO Certificate (Generally Modified Organism)• Residual Solvent Certification (as per USP Chapter 467)• Not of Human/Animal Origin Certificate• Allergen-Free Certificate1. Chowhan, Z.T. and Chi, L.H. (1986); Drug Excipient Interactions Resulting fromPowder Mixing IV: Role of Lubricants and their Effect on In Vitro Dissolution;Journal of Pharmaceutical Sciences, 75: 542-545. doi: 10.1002/jps.26007506042. Gebert, K. Meyer-Bohm, A. Maschke and K. Kolter; Compression Characterizationand Lubricant Sensitivity of Orally Disintegrating Tablets Based on Lubiflash;Pharmaceutical Technology Europe, January 1, 2009, Volume 21, Issue 13. G.K. Bolhuis, C.F. Lerk, H.T. Zijlstra and A.H. de Boer; Pharm. Weekblad 110 317(1975)4. Tommasina Coviello, Antonio Palleschi, Mario Grassi, Pietro Matricardi, GianfrancoBocchinfuso and Franco Alhaique; Scleroglucan: A Versatile Polysaccharide forModified Drug Delivery; Molecules 31, January 2005, 10, 6-33