FMC Alubra brochure8 comp:Layout 1 - FMC BioPolymer
FMC Alubra brochure8 comp:Layout 1 - FMC BioPolymer
FMC Alubra brochure8 comp:Layout 1 - FMC BioPolymer
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>FMC</strong> <strong>BioPolymer</strong>Our lubricant brings more to the tablet.
<strong>Alubra</strong> offers improved API dissolution.Lubricants are a vital part of the tableting manufactureprocess. Formulators have typically selected magnesiumstearate to meet these needs as a familiar, well-establishedlubricant.Given today’s challenges in formulating tablets, it is importantto ensure that the lubricant choice does not have a negativeimpact on disintegration and dissolution. Scientific evidencedemonstrates that magnesium stearate can negatively impactdisintegration and dissolution 1 . Formulators need to considerthese variables before selecting the appropriate lubricant fortheir formulation.<strong>Alubra</strong> has important solubility benefits that can be usedto advantage in developing poorly soluble drugs, orallydisintegrating tablets (ODTs) 2 , and effervescent dosage forms,which all require more water-soluble type lubricants thanmagnesium stearate.Product Benefits:Compared with the widely used lubricantmagnesium stearate, <strong>Alubra</strong> offers:Enhanced dissolutionCompatibility with a range of APIsFlexibility in blendingImproved <strong>comp</strong>actabilitySimilar lubrication performanceBetter tablet quality<strong>Alubra</strong> delivers betterThe chemistry of <strong>Alubra</strong> provides importantadvantages.In addition to offering improved water dispersability of themolecule, the fumarate moiety of <strong>Alubra</strong> increases themelting temperature, which allows greater functionality at highpress speeds when <strong>comp</strong>ared to magnesium stearate. Thestearate chain of <strong>Alubra</strong> maintains the lubricity of the <strong>comp</strong>ound,supporting low ejection forces.Chemical structure of <strong>Alubra</strong> sodium stearyl fumarateHOOTypical <strong>Alubra</strong> PropertiesMolecular Formula: C 22 H 39 NaO 4Formula Weight: 390.53Melting Point:220-240° CRecommended Use level: 0.5-2.0% of FormulationChemical name:2-Butenedioicacidiconoctadecyl ester,sodium saltAcidity:pH 8.3 (5% water solutionat 90° C)Solubility:acetone practically insolubleParticle morphology of <strong>Alubra</strong> ethanol practically insolublemethanol slightly solubleWater 1:5 90° CWater 1:20,000 25° CDensity (bulk):0.3 to 0.5 g/ccOO-Na +Density (tapped):0.4 to 0.6 g/ccEINECS #: 223-781-1CID #: 23665634CAS No. 4070-80-8
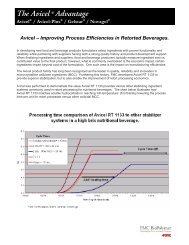
<strong>Alubra</strong> enhances dissolution of poorlysoluble APIs.Many of the new generation APIs are poorly soluble, creatingnew challenges for tablet manufacturers.While it is today’s most widely used lubricant, magnesiumstearate is very hydrophobic and has detrimental effects onthe dissolution profile of APIs 1 , especially poorly soluble APIs 3 .As a highly soluble lubricant, <strong>Alubra</strong> sodium stearyl fumaratehas shown improved disintegration times in hydrophilic matrixformations 4 and ODTs 2 when <strong>comp</strong>ared to magnesium stearate.Magnesium stearate tends to form ‘envelopes’ around APIparticles, slowing tablet disintegration and API dissolution, asopposed to the more hydrophilic <strong>Alubra</strong> , which promotesfaster API dissolution of poorly soluble APIs.% API released12010080604020Comparative Dissolution Propertiesof 500mg Acetaminophen Tablets98% acetaminophen + 2% <strong>Alubra</strong>(Hardness 108N; relative density 85.35%)98% acetaminophen + 2% MgSt(Hardness 119N; relative density 87.85%)0 5 10 15 20 25 30 35 40 45 50 55 60 65Time (min)Source: <strong>FMC</strong> <strong>BioPolymer</strong> 2010dissolution and much more.<strong>Alubra</strong> offers the lubrication performanceyou want.During manufacture, an extensive amount of friction is generatedbetween the tablet and the surfaces of the die and punch asthe tablet is ejected. This can impart considerable amounts ofstrain and shear to a tablet, resulting in defects such as sticking,capping, and lamination.Lubricants like magnesium stearate and sodium stearyl fumaratehave a notable effect in reducing ejection forces. The higherthe ejection force, the greater the possibility that costly tabletdefects will occur.Ejection Force (N)300250200150100Comparative Ejection ForcesAvicel ® PH-102 neatAvicel ® PH-102 + 1% <strong>Alubra</strong> Avicel ® PH-102 + 1% MgStThe chemistry of <strong>Alubra</strong> sodium stearyl fumarate is uniquein promoting improved wettability of the tablet while still allowingfor high formulation lubricity, especially at higher tabletingspeeds. Under the high melting temperatures that result,<strong>Alubra</strong> allows prolonged <strong>comp</strong>ression times at higher<strong>comp</strong>ression forces. Test results indicate that <strong>Alubra</strong> is equalto magnesium stearate when it comes to ejection forces.500 5 10 15 20 25 30 35Source: <strong>FMC</strong> <strong>BioPolymer</strong> 2010Compression Force (kN)
<strong>Alubra</strong> provides greater tablet strength.Trials were run on <strong>Alubra</strong> to evaluate its <strong>comp</strong>ressionperformance versus magnesium stearate. The results indicatethat <strong>Alubra</strong> offers superior <strong>comp</strong>ression versus magnesiumstearate across the <strong>comp</strong>lete <strong>comp</strong>ression profile. Furthermore,<strong>Alubra</strong> shows no drop-off in hardness versus magnesiumstearate at higher <strong>comp</strong>ression forces.Hardness (N)500450400350300250200Comparative Tablet HardnessAvicel ® PH-102 neatAvicel ® PH-102 + 1% <strong>Alubra</strong> Avicel ® PH-102 + 1% MgStWhen <strong>comp</strong>actability was measured on a rotary press, theblend using <strong>Alubra</strong> resulted in the hardest tablets, indicatingthat <strong>Alubra</strong> is a better choice than magnesium stearate whenit comes to high-speed tableting, and/or higher <strong>comp</strong>ressionforces.1501005005Source: <strong>FMC</strong> <strong>BioPolymer</strong> 201010 15 20 25 30 35Compression Force (kN)FORMULAIngredient%FormulaAvicel ® PH-102: 99<strong>Alubra</strong> or Magnesium Stearate: 1PROCEDURE• Materials: Avicel ® PH-102 + 1% lubricant(<strong>Alubra</strong> or MgSt) and Avicel ® PH-102 neat• Sieved through 710µm• Blending time 5 minutes• Rotary press: Manesty D4• One set of round, normal concave, D-type,12 mm diameter punches (position #1)• Tablet weight: 500 mg• Rotation speed: 34 RPM• Compression force: 5, 10, 15, 20, 25, 30 kN(for Avicel ® PH-102 neat: 2, 4, 8, 12, 16, 20 kN)tablet strength and quality.<strong>Alubra</strong> meets the highest quality standards.<strong>Alubra</strong> is manufactured under IPEC and cGMP conditions,which <strong>comp</strong>ly with recognized global quality standards. <strong>FMC</strong>’sown quality initiatives are designed to produce a lubricant thatis chemically, physically, and functionally consistent, as wellas <strong>comp</strong>liant with the stringent requirements of all majorpharmacopoeias. <strong>FMC</strong> carries out systematic product evaluationto ensure that <strong>Alubra</strong> continues to meet or surpass thesehigh standards.<strong>Alubra</strong> sodium stearyl fumarate is included in the FDAInactive Ingredient Database for oral capsules and tablets,as well as in the Canadian List of Acceptable Non-MedicinalIngredients.<strong>Alubra</strong> Commercial Information<strong>Alubra</strong> <strong>comp</strong>lies with all major pharmacopoeias:• NF • PH. Eur. • CP• JPE• BPCommercial packaging:• 1 KG (fiber)• 5 KG (fiber)• 20 KG (fiber)Available supporting documentation for <strong>Alubra</strong> :• Specification Sheet• MSDS• COAs• BSE/TSE Certificate• GMO Certificate (Generally Modified Organism)• Residual Solvent Certification (as per USP Chapter 467)• Not of Human/Animal Origin Certificate• Allergen-Free Certificate1. Chowhan, Z.T. and Chi, L.H. (1986); Drug Excipient Interactions Resulting fromPowder Mixing IV: Role of Lubricants and their Effect on In Vitro Dissolution;Journal of Pharmaceutical Sciences, 75: 542-545. doi: 10.1002/jps.26007506042. Gebert, K. Meyer-Bohm, A. Maschke and K. Kolter; Compression Characterizationand Lubricant Sensitivity of Orally Disintegrating Tablets Based on Lubiflash;Pharmaceutical Technology Europe, January 1, 2009, Volume 21, Issue 13. G.K. Bolhuis, C.F. Lerk, H.T. Zijlstra and A.H. de Boer; Pharm. Weekblad 110 317(1975)4. Tommasina Coviello, Antonio Palleschi, Mario Grassi, Pietro Matricardi, GianfrancoBocchinfuso and Franco Alhaique; Scleroglucan: A Versatile Polysaccharide forModified Drug Delivery; Molecules 31, January 2005, 10, 6-33
<strong>FMC</strong> is today’s global leader in high-quality excipients for pharmaceuticals and supplements.As the world’s leading manufacturer of high-quality pharmaceutical and supplement excipients, <strong>FMC</strong> has extensive experiencein formulation and processing. When customers use <strong>Alubra</strong> , they get the benefit of this unparalleled experience, in the formof exceptional technical support.<strong>FMC</strong>’s technical service professionals perform a number of essential tasks for customers, including conducting functionality,stability, and other tests, helping customers find solutions to their processing problems, conducting training for customerpersonnel, and providing access to <strong>comp</strong>rehensive reference libraries.For further information on <strong>FMC</strong>’s outstanding products and services, contact us at 1-800-526-3649 or go to our website atwww.fmc.comSales OfficesUnited StatesPhiladelphia, PAEuropeBrussels, BelgiumAsia-PacificHong KongLatin AmericaMontevideo, UruguaySales/TechnicalAssistance:Tel: 1-215-299-6534Fax: 1-215-299-6669Customer Service:Tel: 1-800-526-3649Fax: 1-215-299-6475Sales/TechnicalAssistance:Tel: +32-2-775-8311Fax: +32-2-775-8300Customer Service:Tel: +353-21-4354-133Fax: +353-21-4353-057Tel: +852-2839-6600Fax: +852-2576-3770Tokyo, JapanTel: +81-3-3402-3739Fax: +81-3-3402-3700Tel/Fax: +5982-6043030Tel/Fax: +5882-6043104Middle EastAmman, JordanTel: +962-5-4618150Fax: +962-5-4618156Patents<strong>FMC</strong> Corporation is owner and/or licensee of several patents related to its products. The products, processes, and uses of such products referred to in this document may be covered byone or more patents or pending applications in the United States and/or other countries. <strong>FMC</strong> does not warrant against any infringement claim arising from the sales and/or use of any<strong>FMC</strong> product in combination with other materials; the use of any <strong>FMC</strong> product in the operation of any process; any <strong>FMC</strong> product manufactured to a customer’s designs or specifications;or any <strong>FMC</strong> product manufactured by any process requested by a purchaser.Product SuitabilityThe information contained in this document (as well as any advice or assistance) is provided by <strong>FMC</strong> only as a courtesy and is intended to be general in nature. Any uses suggestedby <strong>FMC</strong> are presented only to assist our customers in exploring possible applications. <strong>FMC</strong> makes no warranty, express or implied, as to its accuracy or <strong>comp</strong>leteness, or the resultsto be obtained from such information, advice, or assistance. Each customer is solely responsible for determining whether the <strong>FMC</strong> products are suitable for each customer’s intendeduse, and for obtaining any necessary governmental registrations and approvals for such customer’s production, marketing, sale, use and/or transportation of finished goods using orincorporating the <strong>FMC</strong> products.www.fmcbiopolymer.com/pharmaceuticalpharm_info@fmc.com<strong>FMC</strong> logo, <strong>Alubra</strong> and Avicel are trademarks of <strong>FMC</strong> Corporation.© 2010 <strong>FMC</strong> Corporation. All rights reserved. <strong>Alubra</strong>.09.2010-1.PTG