Spring Organic Chemistry Experiment #3
Spring Organic Chemistry Experiment #3
Spring Organic Chemistry Experiment #3
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
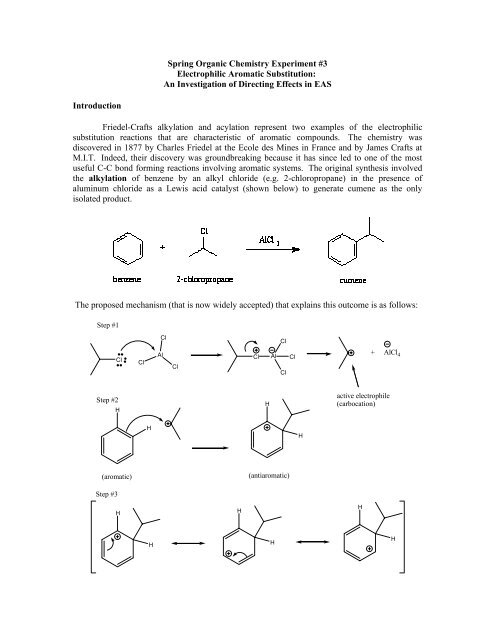
<strong>Spring</strong> <strong>Organic</strong> <strong>Chemistry</strong> <strong>Experiment</strong> <strong>#3</strong>Electrophilic Aromatic Substitution:An Investigation of Directing Effects in EASIntroductionFriedel-Crafts alkylation and acylation represent two examples of the electrophilicsubstitution reactions that are characteristic of aromatic compounds. The chemistry wasdiscovered in 1877 by Charles Friedel at the Ecole des Mines in France and by James Crafts atM.I.T. Indeed, their discovery was groundbreaking because it has since led to one of the mostuseful C-C bond forming reactions involving aromatic systems. The original synthesis involvedthe alkylation of benzene by an alkyl chloride (e.g. 2-chloropropane) in the presence ofaluminum chloride as a Lewis acid catalyst (shown below) to generate cumene as the onlyisolated product.The proposed mechanism (that is now widely accepted) that explains this outcome is as follows:Step #1ClClClClAlClCl Al ClCl+ AlCl 4Step #2HHactive electrophile(carbocation)HH(aromatic)(antiaromatic)Step <strong>#3</strong>HHHHHH
Step #4HClH Cl Al ClClThe entire mechanism occurs in four steps: (1) generation of the active electrophile; (2) attack ofthe elctrophile by the pi electrons of the aromatic ring; (3) resonance stabilization of the resultingintermediate; and (4) regeneration of the aromatic system. The active electrophile in thisalkylation process is a carbocation intermediate that is derived from an alkyl halide. You shouldread pages 679-696 in the Jones text before coming to lab.This groundbreaking work has spurned others to continue the original work of Friedel andCrafts. In particular, Professor George Olah at USC has derived most of the important details thatwe now know about the chemistry of the Friedel-Crafts reaction. In fact, Olah won the 1994Nobel Prize for his pioneering efforts in the electrophilic aromatic substitution chemistryassociated with the Friedel-Crafts reaction. Although this reaction is synthetically useful, it doessuffer from a variety of limitations (see pages 690-691 of Jones).An important variation on the Friedel-Crafts alkylation is called a Friedel-Craftsacylation. In contrast, the electrophile for the Friedel-Crafts acylation is an acylium ionintermediate and it is derived from an acyl halide or an anhydride (i.e. acetyl chloride). The finalproduct obtained in this reaction is an acylated aromatic ring (note: an acyl group contains acarbonyl and typically it’s a ketone).ObjectiveIn this experiment, we will be performing the Friedel-Crafts alkylation of m-xylene using2-chloro-2-methylpropane in the presence of the Lewis acid catalyst, FeCl 3 .
Our goal is to use proton NMR analysis in order to determine the structure of our product.However, we must first consider the possibilities. In other words, what structures are possiblegiven these starting materials and the known mechanism of the reaction? Well, if you knowsomething about the mechanism of the reaction and the effect that ring substituents can have onthe course of further ring substitution, then you should be able to predict ALL of the possibilities(both favorable and unfavorable). I recommend that you check out Chemactivity #29 from thepurple book (in particular, see pages 253-261 and Table 6 on page 259). Once you have thepossibilities in front of you, you should be able to prioritize which ones are most favorable andwhich are least favorable. So, your job (before lab) is to work through the mechanism (as bestyou can) given the information above in order to derive all of the possible products. You shouldthen establish a hypothesis predicting which of the possible products most likely would form.Finally, you should come to lab and conduct the experiment to either confirm or refute yourhypothesis.ProcedurePlace 0.9 mL of m-xylene and 0.75 mL of 2-chloro-2-methylpropane in a dry reactiontube equipped with a rubber septum and the polyethylene tubing that leads to a second tubecontaining a small wad of moist cotton (2-3 drops of water). Cool the mixture in an ice bath andcarefully add 40 mg of anhydrous iron (III) chloride. (Why does everything have to bedry/anhydrous?) Replace the septum and let the reaction proceed. Record your observationscarefully.Once the reaction subsides, remove the ice bath and allow the reaction to warm to roomtemperature. Let the reaction go for an additional 15 minutes and then quench the reaction with1.5 mL of water. Mix the solution well and then remove the aqueous layer with a Pasteurpipette. Repeat this procedure two more times using 1 mL of saturated NaHCO 3 solution,followed by 1 mL of brine. Transfer the remaining solution to a clean reaction tube and dry withanhydrous MgSO 4 .Transfer the dried liquid to yet another reaction tube, add a small boiling chip, and heatthe mixture to its boiling point. Heat so the vapors rise to a level of about 3 cm in the reactiontube. Draw the vapors into a Pasteur pipette and squirt this "distillate" into a final reaction tubethat you a holding in the same hand (instant microscale distillation). Continue until you havedistilled approximately half of the mixture.Analyze your product by preparing a 20-30 mg sample for NMR analysis ( 1 H, 13 C, 13 CDEPT, and 1 H- 1 H COSY) in the usual fashion using CDCl 3 /TMS as the solvent. In addition, usethe Spartan 04 molecular modeling software to build each of your proposed reaction products andcarry out molecular mechanics calculations to determine the relative steric energies for eachproduct. Finally, during lab, work through the mechanism for the Friedel-Crafts acylationreaction (shown above) with your lab partner and discuss.