Systematic review, meta-analysis and economic modelling of ...
Systematic review, meta-analysis and economic modelling of ... Systematic review, meta-analysis and economic modelling of ...
Assessment of diagnostic and prognostic accuracyTABLE 4 Population characteristics of studies of TnTStudy Study typePopulation: age (years) andsex Inclusion criteria Exclusion criteriaTime fromsymptoms(hours)No. ofpatientsBody 2011 46 Single centre, UK Mean age 59, 434 (61%) male Suspected cardiac chest painoccurring within the previous24 hoursChest trauma, renal failurerequiring dialysis, medical conditionnecessitating admission, pregnancy3.5 (median) 713Cete 2010 58 Single centre, Turkey Mean age 57, 163 (73%) male Aged > 18 years presenting tothe ED with typical chest painAtypical chest pain, musculoskeletaltrauma, electrical cardioversionwithin the last 24 hours,musculoskeletal disease, acute orchronic renal failure, liver diseaseNR 224Christ 2011 60 Single centre, Germany Mean age 66, 87 (64%) male Acute chest pain of possiblecoronary originNo other criteria reported 48%< 6 hours137Collinson 2006 62 Multicentre, UK Median age 60, 150 (70%)maleChest pain due to suspectedACS within 24 hoursSTEMI 3 (median) 213Haltern 2010 67 Single centre, Germany Mean age 69, 27/49 (55%)maleIschaemic-type chest pain Age < 18 years, interhospitaltransfer4 (median) 94Keller 2010 71 Multicentre, Germany Mean age 61, 920 (66%) male Aged 18–85 years, with anginapectoris or equivalent symptomsTrauma or major surgery within thelast 4 weeks, pregnancy, intravenousdrug abuse, and anaemia57.6%< 6 hours1386Li 2010 73 Multicentre, China Mean age 64, 163 (72%) male Chest pain for > 30 minutesand < 12 hours, suspected ofMINo other criteria reported 4 (median) 227McCann 2008 76 Multicentre, NorthernIrelandMean age 63, 281 (68%) male Ischaemic-type chest painwithin 24 hoursAge < 18 years, interhospitaltransfer, and previous participationin the study5.3 (median) 415Naroo 2009 78 Single centre, UnitedArab EmiratesAge not reported, 627 (79%)maleTypical chest pain within12 hoursSTEMI, known renal disease NR 791Reichlin 2009 19 Multicentre, international Median age 64, 471 (66%)maleChest pain within 12 hours Terminal kidney failure requiringdialysisNR 718Valle 2008 82 Multicentre, Spain Mean age 65, 287 (68%) male Suspected ACS with symptomsbetween 20 minutes and180 minutes of presentationNo other criteria reported 1.2 (mean) 41922NIHR Journals Library
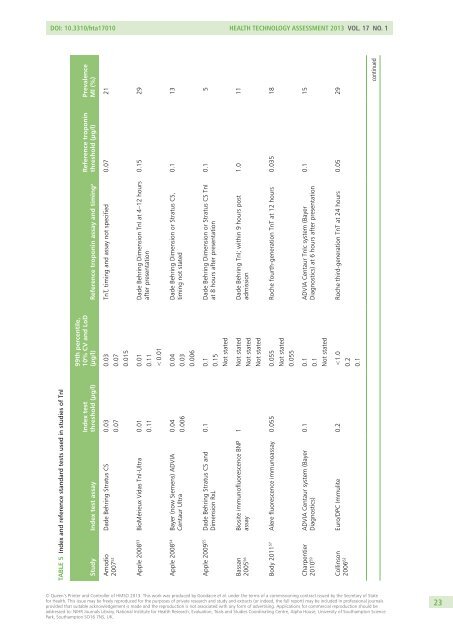
DOI: 10.3310/hta17010 Health Technology Assessment 2013 Vol. 17 No. 1TABLE 5 Index and reference standard tests used in studies of TnIStudy Index test assayIndex testthreshold (µg/l)99th percentile,10% CV and LoDReference troponin(µg/l) Reference troponin assay and timing a threshold (µg/l)PrevalenceMI (%)Amodio Dade Behring Stratus CS 0.032007 52 0.070.030.070.015TnT, timing and assay not specified 0.07 21Apple 2008 53 BioMérieux Vidas TnI-Ultra 0.010.110.010.11< 0.01Dade Behring Dimension TnI at 4–12 hoursafter presentation0.15 29Apple 2008 54 Bayer (now Siemens) ADVIACentaur Ultra0.040.0060.040.030.006Dade Behring Dimension or Stratus CS,timing not stated0.1 13Apple 2009 55 Dade Behring Stratus CS andDimension RxL0.1 0.10.15Not statedDade Behring Dimension or Stratus CS TnIat 8 hours after presentation0.1 5Bassan Biosite immunofluorescence BNP2005 56 assay1 Not statedNot statedNot statedDade Behring TnI; within 9 hours postadmission1.0 11Body 2011 57 Alere fluorescence immunoassay 0.055 0.055Roche fourth-generation TnT at 12 hours 0.035 18Not stated0.055Charpentier ADVIA Centaur system (Bayer2010 59 Diagnostics)0.1 0.10.1Not statedADVIA Centaur TnIc system (BayerDiagnostics) at 6 hours after presentation0.1 15Collinson Euro/DPC Immulite 0.2
- Page 1: Health Technology AssessmentVOLUME
- Page 7: DOI: 10.3310/hta17010 Health Techno
- Page 10 and 11: ContentsAppendix 5 Expected discoun
- Page 13 and 14: DOI: 10.3310/hta17010 Health Techno
- Page 15 and 16: DOI: 10.3310/hta17010 Health Techno
- Page 17 and 18: DOI: 10.3310/hta17010 Health Techno
- Page 19: DOI: 10.3310/hta17010 Health Techno
- Page 22 and 23: BackgroundTABLE 1 Hospital admissio
- Page 24 and 25: Background2. investigation of the c
- Page 26 and 27: BackgroundComputed tomographic coro
- Page 28 and 29: Research questionsiii. the diagnost
- Page 30 and 31: Assessment of diagnostic and progno
- Page 32 and 33: Assessment of diagnostic and progno
- Page 34 and 35: Assessment of diagnostic and progno
- Page 36 and 37: Assessment of diagnostic and progno
- Page 38 and 39: Assessment of diagnostic and progno
- Page 40 and 41: Assessment of diagnostic and progno
- Page 44 and 45: Assessment of diagnostic and progno
- Page 46 and 47: Assessment of diagnostic and progno
- Page 48 and 49: Assessment of diagnostic and progno
- Page 50 and 51: Assessment of diagnostic and progno
- Page 52 and 53: Assessment of diagnostic and progno
- Page 54 and 55: Assessment of diagnostic and progno
- Page 56 and 57: Assessment of diagnostic and progno
- Page 58 and 59: Assessment of diagnostic and progno
- Page 60 and 61: Assessment of diagnostic and progno
- Page 62 and 63: Assessment of diagnostic and progno
- Page 64 and 65: Assessment of diagnostic and progno
- Page 66 and 67: Assessment of diagnostic and progno
- Page 68 and 69: Assessment of diagnostic and progno
- Page 70 and 71: Assessment of diagnostic and progno
- Page 72 and 73: Assessment of diagnostic and progno
- Page 74 and 75: Assessment of diagnostic and progno
- Page 76 and 77: Assessment of diagnostic and progno
- Page 78 and 79: Assessment of diagnostic and progno
- Page 80 and 81: Assessment of diagnostic and progno
- Page 82 and 83: Assessment of diagnostic and progno
- Page 84 and 85: Assessment of diagnostic and progno
- Page 86 and 87: Assessment of diagnostic and progno
- Page 88 and 89: Assessment of diagnostic and progno
- Page 91 and 92: DOI: 10.3310/hta17010 Health Techno
DOI: 10.3310/hta17010 Health Technology Assessment 2013 Vol. 17 No. 1TABLE 5 Index <strong>and</strong> reference st<strong>and</strong>ard tests used in studies <strong>of</strong> TnIStudy Index test assayIndex testthreshold (µg/l)99th percentile,10% CV <strong>and</strong> LoDReference troponin(µg/l) Reference troponin assay <strong>and</strong> timing a threshold (µg/l)PrevalenceMI (%)Amodio Dade Behring Stratus CS 0.032007 52 0.070.030.070.015TnT, timing <strong>and</strong> assay not specified 0.07 21Apple 2008 53 BioMérieux Vidas TnI-Ultra 0.010.110.010.11< 0.01Dade Behring Dimension TnI at 4–12 hoursafter presentation0.15 29Apple 2008 54 Bayer (now Siemens) ADVIACentaur Ultra0.040.0060.040.030.006Dade Behring Dimension or Stratus CS,timing not stated0.1 13Apple 2009 55 Dade Behring Stratus CS <strong>and</strong>Dimension RxL0.1 0.10.15Not statedDade Behring Dimension or Stratus CS TnIat 8 hours after presentation0.1 5Bassan Biosite immun<strong>of</strong>luorescence BNP2005 56 assay1 Not statedNot statedNot statedDade Behring TnI; within 9 hours postadmission1.0 11Body 2011 57 Alere fluorescence immunoassay 0.055 0.055Roche fourth-generation TnT at 12 hours 0.035 18Not stated0.055Charpentier ADVIA Centaur system (Bayer2010 59 Diagnostics)0.1 0.10.1Not statedADVIA Centaur TnIc system (BayerDiagnostics) at 6 hours after presentation0.1 15Collinson Euro/DPC Immulite 0.2