Liquid-Solid Interface I. Electrical Double Layer.
Liquid-Solid Interface I. Electrical Double Layer.
Liquid-Solid Interface I. Electrical Double Layer.
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
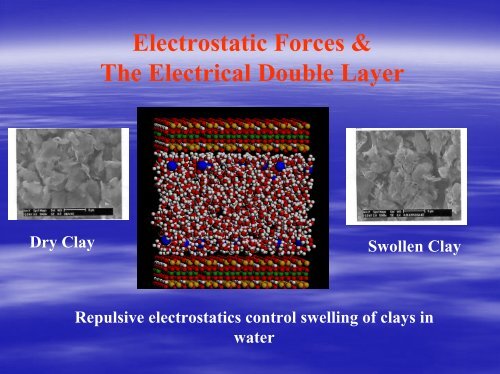
Electrostatic Forces &The <strong>Electrical</strong> <strong>Double</strong> <strong>Layer</strong>Dry ClaySwollen ClayRepulsive electrostatics control swelling of clays inwater
<strong>Liquid</strong>-<strong>Solid</strong> <strong>Interface</strong>; Colloids• Separation techniques such as :• column chromatography, HPLC, PaperChromatography, TLC• They are examples of the adsorption ofsolutes at the liquid solid interface.• <strong>Liquid</strong> solid interfaces can be foundeverywhere and in any form and size, fromelectrode surfaces to ship hulls.
<strong>Liquid</strong>-<strong>Solid</strong> <strong>Interface</strong>; Colloids• Not so obvious examples for a solid-liquidliquidinterface is a colloid particle.• Colloids are widely spread in daily use:• Paint• Blood• Air pollution
<strong>Liquid</strong>-<strong>Solid</strong> <strong>Interface</strong>; Colloids• A very sensitive method to measure theamount of adsorbed material is by using aQCM (quartz crystal microbalance)
<strong>Liquid</strong>-<strong>Solid</strong> <strong>Interface</strong>; Colloids• Adsorption at low solute concentration:• These isotherms can be either fitted by thelangmuir isotherm• Or the freundlich isotherm
<strong>Liquid</strong>-<strong>Solid</strong> <strong>Interface</strong>; Colloids• Stearic acid is adsorbed onto carbon blackdifferently in different solvents:
<strong>Liquid</strong>-<strong>Solid</strong> <strong>Interface</strong>; Colloids• Different chain lengthes will showdifferences in adsorption behaviour:
<strong>Liquid</strong>-<strong>Solid</strong> <strong>Interface</strong>; Colloids• Traube´s s rule:
<strong>Liquid</strong>-<strong>Solid</strong> <strong>Interface</strong>; Colloids• Composite adsorption isotherms:
Intermolecular ForcesInteraction Force/Radius (mN/m)6543210-1-2van der WaalsElectrostaticStericDepletionHydrophobicSolvation0 10 20 30 40 50Separation Distance (nm)Repulsive Forces(Above X-axis)Attractive Forces(Below X-axis)
Electrostatic Forces &The <strong>Electrical</strong> <strong>Double</strong> <strong>Layer</strong>FlagellaE-Coli demonstrate tumbling & locomotive modes of motion in thecell to align themselves with the cell’s rear portionFlagella motion is propelled by a molecular motor made ofproteins – Influencing proton release through protein is a keymolecular approach to prevent E-Coli induced diarrheaMingming Wu, Cornell University (animation)Berg, Howard, C. Nature 249: 78-79, 1974.
Electrostatic Forces & van der Waals Forcesjointly influence Flocculation / CoagulationSuspension of Al 2 O 3 atdifferent solution pHCritical for Water Treatment Processes
Electrostatic Forces &The <strong>Electrical</strong> <strong>Double</strong> <strong>Layer</strong>1) Sources of interfacial charge2) Electrostatic theory: The electrical double layer3) Electro-kinetic Phenomena4) Electrostatic forces
SOURCES OF INTERFACIAL CHARGE• Immersion of some materials in an electrolytesolution. Two mechanisms can operate.(1) Direct Ionization of surface groups.HOMHO OOHOOMOHOOO H- M + H O 2OOO(2) Specific ion adsorptionOH+ + +OMOO
SURFACE CHARGE GENERATION (cont.)(3) Differential ion solubilitySome ionic crystals have a slight imbalancein number of lattice cations or anions onsurface, eg. AgI, BaSO 4 , CaF 2 , NaCl, KCl(4) Substitution of surface ionseg. lattice substitution in kaolinHOSiHOOAlOOSiOO OHSiO OH
ELECTRICAL DOUBLE LAYERSΨ 0--------++SOLVENT MOLECULES-+COUNTER IONS-++x--OHPCO IONSHelmholtz (100+ years ago) proposed that surface chargeis balanced by a layer of oppositely charged ions
Ψ 0---------Gouy-Chapman Model (1910-1913)++ +--++ +--xDiffusion plane• Assumed Poisson-Boltzmann distribution of ions from surface• ions are point charges• ions do not interact with each other• Assumed that diffuse layer begins at some distance from thesurface
Ψ ζΨ 0Stern (1924) / Grahame (1947) Model-------------Gouy/Chapman diffuse double layer and layer of adsorbed charge.Linear decay until the Stern plane.++Stern PlaneDifusion layer++Shear Plane+Gouy Plane--++-Bulk Solution---x
Stern (1924) / Grahame (1947) ModelIn different approaches the linear decay is assumed to be until the shearplane, since there is the barrier where the charges considered static. In thiscourese however we will assume that the decay is linear until the Stern plane.Ψ ζΨ 0-----------++OHPDifusion layer++++Shear Plane+Gouy Plane--++-Bulk Solution--x-
POISSON-BOLTZMANN DISTRIBUTION1 st Maxwell law (Gauss law): “The total of the electric flux out of aclosed surface is equal to the charge enclosed divided by the permittivity”∇ ⋅ E=( r)ρε εElectric field is the differential of the electric potentialE→(r)= −∇ ⋅ Ψ→Combining the two equations we get:∇ ⋅ Ψ rWhich for one dimension becomes:→→()0r() rρ(r= −ε ε2 )2d ΨdxAssuming Boltzmann ion distribution:0( x) ρ( x)2= −n( x) ρ( x) 1−ZeΨkT Zen(−ZΨekT Z e kT= − = − Z nee = − e − e )2d Ψi Ψ2∑ idx εε0εε0 iεε0εε0rρDefinitionsE: Electric filedΨ: Electric potentialρ: Charge densityE Q : Energy of the ionx, r : DistanceBoltzmann ion distributionEeZΨQ−−kTkT( x) e = n Zee= ρ 00
POISSON-BOLTZMANN DISTRIBUTIONd2dxψ2=2Zensinh⎛⎜ε ε ⎝r0ZeψkTZ = electrolyte valence,e = elementary charge (C)n = electrolyte concentration(#/m 3 )ε r = dielectric constant of mediumε 0 = permittivity of a vacuum (F/m)k = Boltzmann constant (J/K)T = temperature (K)• Poisson-Boltzmann distribution describes the EDL• Defines potential as a function of distance from a surface• ions are point charges• ions do not interact with each other⎞⎟⎠
POISSON-BOLTZMANN DISTRIBUTIONDebye-Hückel approximationZeΨkT
DOUBLE LAYER FOR MULTIVALENTELECTROLYTE: DEBYE LENGTHDebye-Hückel parameter (κ) describes the decay length1/ 22 n⎛ e2⎞κ = ⎜ ∑C ⎟iZi⎝ εrε0kTi=1 ⎠Z i = electrolyte valencee = elementary charge (C)C i = ion concentration (#/m 3 )n = number of ionsε r = dielectric constant of mediumε 0 = permittivity of a vacuum (F/m)k = Boltzmann constant (J/K)T = temperature (K)κ -1 (Debye length) has units of length
POISSON-BOLTZMANN DISTRIBUTIONExact SolutionFor 0.001 M 1-1 electrolyteSurface Potential (mV)Surface Potential (mV)ZeΨkT 1
DEBYE LENGTH AND VALENCYDebye Length κ -1 , (nm)1009080706050403020101-1 electrolyte2-2 electrolyte3-3 electrolyte010 -5 10 -4 10 -3 10 -2 10 -1 10 0 10 1Electrolyte Concentration (M)• Ions of higher valence are more effective in screeningsurface charge.
ZETA POTENTIALPoint of Zero Charge (PZC) - pH at which surface potential = 0Isoelectric Point (IEP) - pH at which zeta potential = 0Question: What will happen to a mixed suspension of Alumina andSi 3 N 4 particles in water at pH 4, 7 and 9?
ZETA POTENTIAL-- Effect of Ionic Strength --Zeta Potential (mV)50403020100-10-20-30-40-50AluminaIncreasingI.S.1 2 3 4 5 6 7 8 9 1 0 1 1pH
SPECIFIC ADSORPTION•Free energy decrease upon adsorption greater than predicted byelectrostatics• Have the ability to shift the isoelectric point v and reverse zetapotential• Multivalent ions: Ca +2 , Mg +2 , La +3 , hexametaphosphate, sodiumsilicate• Self-assembling organic molecules: surfactants, polyelectrolytes++++++ + ++++2+ +- - - - -++
SPECIFIC ADSORPTIONCa 2+PO 43-pHMultivalent cations shift IEP to right (calcite supernatant)Multivalent anions shift IEP to left (apatite supernatant)Amankonah and Somasundaran, Colloids and Surfaces, 15, 335 (1985).
ELECTROKINETIC PHENOMENA• Electrophoresis - Movement of particle in a stationaryfluid by an applied electric field.• Electro-osmosis - Movement of liquid past a surface byan applied electric field• Streaming Potential - Creation of an electric field as aliquid moves past a stationary charged surface• Sedimentation Potential - Creation of an electric fieldwhen a charged particle moves relative to stationary fluid
ZETA POTENTIAL MEASUREMENT• Electrophoresis - ζ determined by the rate of diffusion(electrophoretic mobility) of a charged particle in an applied DCelectric field.• PCS - ζ determined by diffusion of particles as measured byphoton correlation spectroscopy (PCS) in applied field• Acoustophoresis - ζ determined by the potential created by aparticle vibrating in its double layer due to an acoustic wave• Streaming Potential - ζ determined by measuring the potentialcreated as a fluid moves past macroscopic surfaces or a porousplug
ZETA POTENTIAL MEASUREMENTElectrophoresisSmoluchowski Formula (1921)assumed κa >> 1 κ = Debye parametera = particle radius- electrical double layer thickness much smaller thanparticlev =µΕε=rεηε0rζεη0Εζv = velocity,ε r = media dielectric constantε 0 = permittivity of free spaceζ = zeta potential, E = electric fieldη = medium viscosityµ E = electrophoretic mobility
ZETA POTENTIAL MEASUREMENTElectrophoresisHenry Formula (1931)expanded for arbitrary κa, assumed E field does notalter surface charge-low σ 0vµ2εrε=3ηΕ0ζf2εrε=3η01ζf( κa)Ε1( κa)v = velocityε r = media dielectric constantε 0 = permittivity of free spaceζ = zeta potentialη = medium viscosityE = electric fieldµ E = electrophoretic mobilityHunter, Foundations of Colloid Science, p. 560
ZETA POTENTIAL MEASUREMENTStreaming Potentialexp(Zeζ/κa2kT)
Combined Effects of van der Waals andElectrostatic ForcesDLVO TheoryDLVO – Derjaguin, Landau, Verwey and OverbeekBased on the sum of van der Waals attractive potential anda screened electrostatic repulsion potential arising betweenthe “double layer potential” screened by ions in solution.The total interaction energy U of the system is:2AR 64πkTRnγU ( x)= − +exp212xκVan der Waals(Attractive force)Electrostatics(Repulsive force)( −κx)
DLVO Theory2AR 64πkTRnγU ( x)= − +exp212xκ( −κx)A = Hamakar’s constantR = Radius of particlex = Distance of Separationk = Boltzmann’s constantT = Temperaturen = bulk ion concentrationκ= Debye parameterγ =⎛tanh⎜⎝zeψ⎞⎟4kT⎠z = valency of ione = Charge of electronΨ = Surface potential
DLVO Theory100 nm Alumina, 0.01 M NaCl, Ψ zeta=-20 mVFor short distances of separation between particles
DLVO TheoryHard Sphere Repulsion(< 0.5 nm)No Salt addedJ/mEnergy Barrierx (distance)Secondary Minimum(Flocculation)Primary Minimum(Coagulation)
Discussion: Flocculation vs. CoagulationThe DLVO theory defines formally (and distinctly), the ofteninter-used terms flocculation and coagulationFlocculation:• Corresponds to the secondary energy minimum at large distances ofseparation• The energy minimum is shallow (weak attractions, 1-2 kT units)• Attraction forces may be overcome by simple shakingCoagulation:• Corresponds to the primary energy minimum at short distances ofseparation upon overcoming the energy barrier• The energy minimum is deep (strong attractions)• Once coagulated, particle separation is almost impossible
Hard Sphere Repulsion(< 0.5 nm)J/mEffect of SaltEnergy BarrierNo Salt addedUpon Salt additionx (distance)Secondary Minimum(Flocculation)Primary Minimum(Coagulation)Addition of salt reduces the energy barrier of repulsion.How?
Secondary Minimum: Real System100 nm Alumina, Ψ zeta=-30 mV
Discussion on the Effect of SaltThe salt reduces the EDL thickness by charge screeningReduces the energy barrier (may induce coagulation)Also increases the distance at which secondary minimumoccurs (aids flocculation)Since increased salt concentration decreases κ -1 (ordecreases electrostatics), at the Critical Salt ConcentrationU(x) = 0
Effect of Salt Concentration and TypeAR−12H+64πkTRnγ2κ2exp( −κH) = 0H: Distance of separation at critical salt concentrationAt critical salt concentration, κH = 1.Upon simplification, we get:nα1z6Schultz – Hardy Rule:n: ConcentrationZ: ValenceConcentration to induce rapid coagulation variesinversely with charge on cation
Effect of Salt Concentration and TypeFor As 2 S 3 sol, KCl: MgCl 2 : AlCl 3 required to induceflocculation and coagulation varies by a simpleproportion 1: 0.014: 0.0018The DLVO theory thus explains why alum (AlCl 3 ) andpolymers are effective (functionality and cost wise) toinduce flocculation and coagulation
pH and Salt Concentration EffectAgglomerateDispersionDispersionStabilitydiagram forSi 3 N 4 (M11)particles asproduced fromcalculations(IEP 4.4)assuming 90%probability ofcoagulation forsolid formation.
REMARKS-- hydrophobic and solvation forces --• Due to the number of fitting parameters (ψ 0, A 132,spring constant, I.S.) and uncertainty in force laws (C.C.vs C.P., retardation) hydrophobic forces often invokedto explain differences between theory and experiment.• Because hydrophobic forces involve the structure of thesolvent, the number of molecules to be considered in theinteraction is large and computer simulation has onlybegun to approach this problem.• Widely accepted phenomenological models ofhydrophobic forces still need to be developed.