Enantioselective Synthesis of Axial Chiral Allenes - The Stoltz Group
Enantioselective Synthesis of Axial Chiral Allenes - The Stoltz Group
Enantioselective Synthesis of Axial Chiral Allenes - The Stoltz Group
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
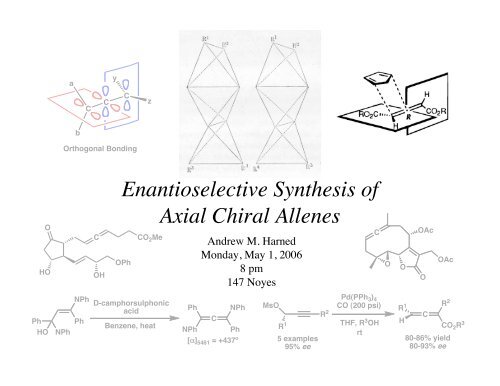
ayCCCzbOrthogonal Bonding<strong>Enantioselective</strong> <strong>Synthesis</strong> <strong>of</strong>OHOCOHOPhCO 2 Me<strong>Axial</strong> <strong>Chiral</strong> <strong>Allenes</strong>Andrew M. HarnedMonday, May 1, 20068 pm147 NoyesCOOOAcOOAcPhHONPhNPhPhD-camphorsulphonicacidBenzene, heatPhNPhCNPh Ph[α] 5461 = +437ºMsOR 15 examples95% eeR 2Pd(PPh 3 ) 4CO (200 psi)THF, R 3 OHrtR 1HCR 280-86% yield80-93% eeCO 2 R 3
<strong>Axial</strong> <strong>Chiral</strong> <strong>Allenes</strong>: Useful Curiosities1) <strong>The</strong> basics <strong>of</strong> <strong>Allenes</strong> and <strong>Axial</strong> <strong>Chiral</strong>itya) A brief history <strong>of</strong> allenesb) Spectroscopyc) Types <strong>of</strong> chiral allenesd) General axial chiralitye) Natural products with axial chiral allenesf) <strong>Allenes</strong> as Pharmaceutical Agents2) Methods <strong>of</strong> Synthesizing <strong>Axial</strong>ly <strong>Chiral</strong> <strong>Allenes</strong>a) Propargyl alcohol derivativesb) Elimination from allylic compoundsc) Stiochiometric chiral reagentsc) Kinetic resolutiond) Catalytic methods3) ConclusionsGeneral Allene Reviews:(General reviews) Taylor, D. R. Chem. Rev. 1967, 67, 317-359. Pasto, D. J. Tetrahedron 1984, 40, 2805-2827.(Electrophilic additions to allenes) Smadja, W. Chem. Rev. 1983, 83, 263-320.(<strong>Synthesis</strong> with organometallics) Krause, N.; H<strong>of</strong>fmann-Röder, A. Tetrahedron 2004, 60, 11671-11694.(<strong>Synthesis</strong> <strong>of</strong> allenic esters) Miesch, M. <strong>Synthesis</strong> 2004, 746-752.(Pd reactions <strong>of</strong> allenes) Zimmer, R.; Dinesh, C. U.; Nandanan, E.; Hkan, F. A. Chem. Rev. 2000, 100, 3067-3125.(Selective reactions with transition metals) Hashmi, A. S. K. Angew. Chem. Int. Ed. 2000, 39, 3590-3593.Schuster, H. F.; Coppola, G. M. <strong>Allenes</strong> in Organic <strong>Synthesis</strong>; Wiley & Sons: New York, 1984.<strong>The</strong> Chemistry <strong>of</strong> the <strong>Allenes</strong>; Landor, S. R., Ed.; Academic Press: New York, 1982.<strong>The</strong> Chemistry <strong>of</strong> Ketenes, <strong>Allenes</strong> and Related Compounds; Patai, S., Ed.; <strong>The</strong> Chemistry <strong>of</strong> Functional <strong>Group</strong>s; Wiley & Sons: New York, 1980.Modern Allene Chemistry; Krause, N., Hashmi, A. S. K., Eds.; Wiley-VCH: Weinheim, 2004.Bruneau, C.; Renaud, J.-L. <strong>Allenes</strong> and cumulenes. In Comprehensive Organic Functional <strong>Group</strong> Transformations II; Katritzky, A. R., Taylor, R. J. K., Eds.;Elsevier: Oxford, 2005.
A Brief History <strong>of</strong> <strong>Allenes</strong>1874-1875: Jacobus H. van't H<strong>of</strong>f predicts the correct structures <strong>of</strong> alenes and cumulenes as well as theiraxial chirality (La Chemie dans l'Espace, Bazendijk: Rotterdam, 1875).1887: In an attempt to prove the nonexistence <strong>of</strong> these compounds, B. S. Burton and H. von Pechmannreport the first synthesis <strong>of</strong> an allene (Ber. Dtsch. Chem. Ges. 1887, 20, 145-149). <strong>The</strong> paucity <strong>of</strong> analyticaltechniques make it very difficult to distinguish from the corresponding alkynes, and the structure is not proven(E. R. H. Jones, G. H. Mansfield, M. L. H. Whiting J. Chem. Soc. 1954, 3208-3212) for almost 70 years!1924: First naturally occuring allene, pyrethrolone, characterized by H. Staudinger and L. Ruzicka (Helv. Chim. Acta 1924, 7, 177).HOOC Me PyrethroloneMe1935: First axially chiral allene synthesized by P. Maitland and W. H. Mills (Nature 1935, 135, 994; J. Chem. Soc. 1936, 987-998).PhHONPhNPhPhD-camphorsulphonicacidBenzene, heatPhNPhCNPhPh[α] 5461 = +437º1952?: First cyclic allenes synthesized by A. T. Blomquist, R. E. Burge, Jr. and A. C. Sucsy (J. Am. Chem. Soc. 1952, 74, 3636-3642; J. Am. Chem. Soc. 1952, 74, 3643-47). Ring sizes above nine can be isolated. 1,2-Cyclooctadiene can be detected in smallamounts at low temperatures. Ring sizes <strong>of</strong> seven or below cannot be isolated or detected. <strong>The</strong>ir intermediacy has been determinedthrough trapping/degradation studies. M. Regitz and coworkers have described a stable six membered cyclic allene, but is not ahydrocarbon (Angew. Chem. Int. Ed. 2000, 39, 1261-1263).
Structure and Spectroscopy <strong>of</strong> <strong>Allenes</strong>IR Spectroscopy: <strong>The</strong> diagnostic stretch can be found at 2000-1900 cm -1 (C=C=C asymm. stretch) and is usually strong.For comparison, alkynes are at 2260-2100 cm -1 and olefins are at 1690-1635 cm -1 .Only other functional groups near this range are: diazo, cyanates, isocyanates, thiocyanates,isothiocyanates, and ketenes; but are typically at slightly higher wavenumbers.1 H NMR Spectroscopy: Typically in the same area as olefinic protons, but slightly upfield (~4.4 - 4.9 ppm).13 C NMR Spectroscopy: Cα and Cγ are more upfield than typical olefin (~73 - 120 ppm).Cβ is actually slightly more downfield than a ketone! (200-220 ppm)spayCα Cβ CγbzaCCyCzsp 2 TMS COOHON(i-Pr) 2bPhHNOOrthogonal BondingSchultz-Fademrecht, C.; Wibbeling, B.; Fröhlich, R.;Hoppe, D. Org. Lett. 2001, 3, 1221-1224.
Structure and Spectroscopy <strong>of</strong> <strong>Allenes</strong>IR Spectroscopy: <strong>The</strong> diagnostic stretch can be found at 2000-1900 cm -1 (C=C=C asymm. stretch) and is usually strong.For comparison, alkynes are at 2260-2100 cm -1 and olefins are at 1690-1635 cm -1 .Only other functional groups near this range are: diazo, cyanates, isocyanates, thiocyanates,isothiocyanates, and ketenes; but are typically at slightly higher wavenumbers.1 H NMR Spectroscopy: Typically in the same area as olefinic protons, but slightly upfield (~4.4 - 4.9 ppm).13 C NMR Spectroscopy: Cα and Cγ are more upfield than typical olefin (~73 - 120 ppm).Cβ is actually slightly more downfield than a ketone! (200-220 ppm)spaysp 2 TMS CCα Cβ CγbzOyazOOHN(i-Pr) 2bPhHNOOrthogonal BondingSchultz-Fademrecht, C.; Wibbeling, B.; Fröhlich, R.;Hoppe, D. Org. Lett. 2001, 3, 1221-1224.
Types <strong>of</strong> <strong>Chiral</strong> <strong>Allenes</strong>Asymmetric allenes(no element <strong>of</strong> symmetry)ABHD•HHADABBD•HHHAB(S)H•AD D B(R)AB•DAAB•DEDissymmetric allene(C 2 proper axis <strong>of</strong> rotation)AB•ABHAB(S)H•DD A B(S)<strong>Chiral</strong> Allene Reviews: Rossi, R.; Diversi, P. <strong>Synthesis</strong> 1973, 25-36. Runge, W. Stereochemistry <strong>of</strong> <strong>Allenes</strong>. In <strong>The</strong> Chemistry <strong>of</strong> <strong>Allenes</strong>; Landor, S. R., Ed.;Academic Press: New York, 1982; 580-678. Schuster, H. F.; Coppola, G. M. <strong>Allenes</strong> in Organic <strong>Synthesis</strong>; Wiley & Sons: New York, 1984, 1-8. H<strong>of</strong>fmann-Röder, A.; Krause, N. Angew. Chem. Int. Ed. 2002, 41, 2933-2935. Ohno, H.; Nagaoka, Y.; Tomioka, K. <strong>Enantioselective</strong> synthesis <strong>of</strong> <strong>Allenes</strong>. In Modern AlleneChemistry; Krause, N., Hashmi, A. S. K., Eds.; Wiley-VCH: Weinheim, 2004; 141-181.
Types <strong>of</strong> <strong>Chiral</strong> <strong>Allenes</strong>Asymmetric allenes(no element <strong>of</strong> symmetry)ABHD•HHADABBDNot coveredAllene is not stereogenic•HHHAB(S)H•AD D B(R)AB•DAAB•DEDissymmetric allene(C 2 proper axis <strong>of</strong> rotation)AB•ABHAB(S)H•DD A B(S)<strong>Chiral</strong> Allene Reviews: Rossi, R.; Diversi, P. <strong>Synthesis</strong> 1973, 25-36. Runge, W. Stereochemistry <strong>of</strong> <strong>Allenes</strong>. In <strong>The</strong> Chemistry <strong>of</strong> <strong>Allenes</strong>; Landor, S. R., Ed.;Academic Press: New York, 1982; 580-678. Schuster, H. F.; Coppola, G. M. <strong>Allenes</strong> in Organic <strong>Synthesis</strong>; Wiley & Sons: New York, 1984, 1-8. H<strong>of</strong>fmann-Röder, A.; Krause, N. Angew. Chem. Int. Ed. 2002, 41, 2933-2935. Ohno, H.; Nagaoka, Y.; Tomioka, K. <strong>Enantioselective</strong> synthesis <strong>of</strong> <strong>Allenes</strong>. In Modern AlleneChemistry; Krause, N., Hashmi, A. S. K., Eds.; Wiley-VCH: Weinheim, 2004; 141-181.
General <strong>Axial</strong> <strong>Chiral</strong>ityDetermining configurationCCW = (S)4H1CO 2 H3CH 3C 4 H 92HO 2 CC 4 H 9•R = MS = PHCH 31CH 34C 4 H 9H23CO 2 HCCW = (S)Predicting absolution configuration and magnitude <strong>of</strong> rotation (Lowe-Brewster Rule)J. H. Brewster (Topics in Stereochemistry, 1967, 2, 1-72):Through mathematical models, the actual optical rotation <strong>of</strong> optically pure chiral allenes can be calculated. At one time thiswas a very common method to determine approximate optical purity (i.e. % ee). Not very amenable to more complicatedsystems, and has now been supplanted by GC, HPLC, and NMR methods.G. Lowe (J. Chem. Soc., Chem. Commun., 1965, 411-413):<strong>The</strong> absolute configuration can be assignned based on the sign (+ or -) <strong>of</strong> rotation. From this, you can then assign S or R.AXYMost polarizable group A is placed at the top. If Y is more polarizable than X, the allene willbe dextrorotatory (+). If X is more polarizable than Y, the allene will be levorotatory (-).B
General <strong>Axial</strong> <strong>Chiral</strong>ityDetermining configurationCCW = (S)4H1CO 2 H3CH 3C 4 H2 9HO 2 CC 4 H 9•R = MS = PHCH 31CH 343C 4 H 9 CO 2 HH2CCW = (S)Predicting absolution configuration and magnitude <strong>of</strong> rotation (Lowe-Brewster Rule)J. H. Brewster (Topics in Stereochemistry, 1967, 2, 1-72):Through mathematical models, the actual optical rotation <strong>of</strong> optically pure chiral allenes can be calculated. At one time thiswas a very common method to determine approximate optical purity (i.e. % ee). Not very amenable to more complicatedsystems, and has now been supplanted by GC, HPLC, and NMR methods.G. Lowe (J. Chem. Soc., Chem. Commun., 1965, 411-413):<strong>The</strong> absolute configuration can be assignned based on the sign (+ or -) <strong>of</strong> rotation. From this, you can then assign S or R.PhCO 2 HClHPhHO 2 CPhMet-BuH(+)-SH(-)-RH(-)-S
nC 8 H 17HCCO 2 MeH~80% eeInsect Pheromone<strong>Allenes</strong> as Natural ProductsNot just curiosities anymoreNH 3C CO 2BrEtOHHOHHIsolauralleneCBrHHOCOHH"Grasshopper Ketone"OHOCOHHMimulaxanthinHHOCOHOHOHOHOkamuralleneHCBrHCCOOOAcOOAcAcOOOHHHOAcalycixeniolide EAnti-angiogenicCCOOOHHNIsodihydrohistrionicotixinNicotine acetylcholine receptor activeCAbout 150 Natural <strong>Allenes</strong> are known. Most are chiral but not necessarily enantiopure.Krause, N.; H<strong>of</strong>fmann-Röder, A. Allenic Natural Products and Pharmaceuticals. In Modern Allene Chemistry; Krause, N., Hashmi, A. S. K., Eds.; Wiley-VCH:Weinheim, 2004; 997-1039.
<strong>Allenes</strong> as Pharmaceutical AgentsNot just curiosities anymoreHCCO 2 NaOCCO 2 MeCCO 2NH 3OHHOOHOPhOHOH OHAnti-thrombotic agentEnprostilGastric acid inhibitorVitamin B 6 -dependentdecarboxylase inhibitor(suicide substrate)NH2NH2OP(OEt) 2NNNCONNNH COHH COHHH(R)-Cytallene(R)-AdenalleneInhibitors <strong>of</strong> HIV and Hepatitis B replicationMeOSterol biosynthesis inhibitor(AIDS-related pathogen)Krause, N.; H<strong>of</strong>fmann-Röder, A. Allenic Natural Products and Pharmaceuticals. In Modern Allene Chemistry; Krause, N., Hashmi, A. S. K., Eds.; Wiley-VCH:Weinheim, 2004; 997-1039.
<strong>Chiral</strong>ity Transfer from Propargylic AlcoholsA) Copper-Mediated MethodsAlkylationHalogenationB) RearrangementsD) S N 2' reactionsC) Palladium (0)-Mediated Methodsanti-eliminationNuR 3 R 3R 1NuR 1R 2 R 2LGLGR 1R 2CNuR 3syn-eliminationR 1Z NuR 1R 3R 2 OOHZ NuR 2 R 3 R 1CR 2R 3Nu
<strong>The</strong> BeginningsR 3R 1LiCuR 4 2R 2OAcR 1R 2CR 3R 4Rona, P.; Crabbé, P. J. Am. Chem. Soc. 1969, 91, 3289-3292.anti-eliminationR 2 Cu R2R 1HLGMCuR 2 2H R1LGR 1HCR 2 Cu R 2 Red.Elim.HR 1HCR 2HLuche, J.-L.; Barreiro, E.; Dollat, J.-M.; Crabbé, P. Tetrahedron Lett. 1975, 4615.Dollat, J.-M.; Luche, J.-L.; Crabbé, P. J. Chem. Soc., Chem. Comm. 1977, 761-762.Many studies with steriodal stystems. Complications due to racemization <strong>of</strong> product by dialkyl cuprates.
Proparglyic Estersnonsteriodal, acyclic allenesR 1HLGR 1 = Ph, t-Bu, octyl, MeLG = OS(O)Me or OSO 2 MeR 2 CuBr⋅MgBr⋅LiBr[{RCuX}M]R 1HCR 2H70-96% yield>98% anti-selectivityHR 1LG[R 2 CuX][MgX]HLGR 1R 2CuXHLGR 1R 2CuX- LGR 1HCR 2 IIICu R 2H- Cu I XLCuRR 1HOd xz p xπ π*C C HR 1HCR 2Hσ*Elsevier, C. J.; Vermeer, P. J. Org. Chem. 1989, 54, 3726-3730.Corey Tetrahedron Lett. 1984, 25, 3063-3066
Proparglyic EstersD-Mannitol4 steps62%TBDPSOOHCBr 4PPh 3 , PyrTHF97%POBrCu388%CO 2 MePOHCH3CO 2 Me(R)>94% ee[α] 25 D -63.8° (c = 0.43, MeOH)>97% eeTsCl, Et 3 NCH 2 Cl 299%POOTsCuCO 2 Me3POHC96%COH3 2 Me(S)>94% ee[α] 25 D +65.0° (c = 0.34, MeOH)TBDPSOHCH3CO 2 MeTBAFTHF98%HOCHHCO 2 Me[α] 25 D -53.4° (c = 0.31, CHCl 3 )3Antifungal constituent fromSapium japonicumlit. [α] 12 D -51.3° (c 0.94, CHCl 3 )Gooding, O. W.; Beard, C. C.; Jackson, D. Y.; Wren, D. L.; Cooper, G. F. J. Org. Chem. 1991, 56, 1083-1088.
Enantioenriched Propargyl AlcoholsOPOC 7 H 15OP = OSit-Bu 2 Ph(R)-Alpine boraneTHF, 20 °C63%PO88% eeCBrOH4 , PPh 3Pyr, THF, 20 °CC 7 H 63%15POBrC 7 H 15LiBr, Cu 2 I 2MeMgBrTHF, 0 °C80%POMeC52% eeHC 7 H 15C 8 H 17 ON NMeOSliquid crystalline propertiesCHC 7 H 15Zab, K.; Kruth, H.; Tschierske, C. J. Chem. Soc., Chem. Comm. 1996, 977-978.O2.5 mol% [(p-cymene)RuCl 2 ] 2(S,S)-TsDPEN, KOH, i-PrOHOHa) BuLi,THF, -78 °Cb) MsClCPh75%Phc) MeCuCNMgBr71%MePh>95% ee>95% eeBuisine, O.; Aubert, C.; Malacria, M. <strong>Synthesis</strong> 2000, 985-989.
Enantioenriched Propargyl AlcoholsOPOC 7 H 15OP = OSit-Bu 2 Ph(R)-Alpine boraneTHF, 20 °C63%PO88% eeCBrOH4 , PPh 3Pyr, THF, 20 °CC 7 H 63%15POBrC 7 H 15LiBr, Cu 2 I 2MeMgBrTHF, 0 °C80%POMeC52% eeHC 7 H 15C 8 H 17 ON NMeOSliquid crystalline propertiesCHC 7 H 15Zab, K.; Kruth, H.; Tschierske, C. J. Chem. Soc., Chem. Comm. 1996, 977-978.CMePhBuLi, Ph 2 P(O)ClTHF, -78 to 50 °C78%CPhMePPh 2OCpCo(CO) 2THF, Δ, hνquant.CpCoHPh 2 P MeO>95% eePhBuisine, O.; Aubert, C.; Malacria, M. <strong>Synthesis</strong> 2000, 985-989.
Enantioenriched Propargyl AlcoholsH 17 C 8O(S)-BINAL-HOH91% C 8 H 1795% eeMsClTEADCM, 0 ºC95%C 8 H 17OMsTMSLiBr, CuBr, THF-60 ºC to rt95%MgClH 17 C 8HCTMSBrummond, K. M.; Kerekes, A. D.; Wan, H. J. Org. Chem. 2002, 67, 5156-5163.Cp 2 ZrCl 2 , BuLiCO, 20 psiH 17 C 8OTHF-78 ºC to rt to 0 ºC39% TMS85% eeH1)PhMeCHO+PhHO NMe2Zn(OTf) 2PhMe, 23 ºC2) MsCl, TEACH 2 Cl 2 , -40 ºC80%OMs99% eePh1) NBocCuCNLi82%2) TMSOTfCH 2 Cl 2 , -40 ºC99%HPhCMeHN83% eeDieter, R. K.; Yu, H. Org. Lett. 2001, 3, 3855-3858.
Enantioenriched Cyclic <strong>Allenes</strong>OAcnA, n = 1B, n = 2Lipase(Mucor miehei)PhosphateBufferOAcn(S)-A-OAc40% (64% ee)+OHn(R)-A-OH43% (91% ee)(S)-B-OAc50% (60% ee)(R)-B-OH20% (90% ee)OAcOAcRMgXCRMgXC(91% ee)CuBr, LiBrTHF, -70 ºCR = Bu, i-Pr, t-Bu(+)R(60% ee)CuBr, LiBrTHF, -70 ºCR = Bu, i-Pr, t-Bu(-)(60% ee)R% ee determined for R = Bu, t-Bu by GC,Enantioseparation not achieved for othersZelder, C.; Krause, N. Eur. J. Org. Chem. 2004, 3968-3971.
Silyl CupratesOHCOBnOTBSCN1a) HCCMgBr, DCM-78 to 0 °C, 89%b) Separate 2:1 dr2) Ac 2 O, TEA, DMAPDCM, 98%orDEAD, PPh 3 , HOAcPyr, THF-45 °C to rt, 86%OBn1) (Me 2 PhSi) 2 Cu(CN)Li 2THF, -96 °C, 84%OTBSAcO H 2) DIBALH, PhMe-78 to 0 °C, 78%CNMe 2 PhSiHCHOBnCHOOTBSOBrONPPh 3mesitylene50 °C then refluxArNHCSiPhMe 2HHOBn2) TBAF, THF, 0 °C63% overallBrNHOBnOTBSOTBSOOOOMeON H(-)-coccinineOHJin, J.; Weinreb, S. M. J. Am. Chem. Soc. 1997, 119, 2050-2051.For an alternative approach to allenyl silanes, see: Guintchin, B. K.;Bienz, S. Organometallics 2004, 23, 4944-4951.
Propargylic SilanesTMSHOMsMeHSiCl 3 , CuClDIEA, RCHODMFTMSCROHMeH+TMSMeOHR55-92% yield89:11 to 98:2>95% chirality transferTMSOMsMeH"CuSiCl 3 "TMSMeHCuXSiCl 3TMSCXCuSiCl 3MeHreductiveelimination- CuClTMSMeHSiCl 3TMSCl 3 SiCMeHRCHORCHOTMSCROHMeHTMSMeRHOHSiCl 3TMSCl 3 SiCOMeHHRTMSMeOHRThis Work: Marshall, J. A.; Adams, N. D. J. Org. Chem. 1997, 62, 8976-8977.For similar chemistry with stannanes, see: Marshall, J. A.; Yu, R. H.; Perkins, J. F. J. Org. Chem. 1995, 60, 5550-5555.
S N 2' reactionCO 2 MeDIBAL-HCHOHHOOenantiomerically pureCH 2 Cl 20 ºC80%HOHOHOOOFuruichi, N.; Hara, H.; Osaki, T.; Mori, H.; Katsumura, S.Angew. Chem. Int. Ed. 2002, 41, 1023-1026.HOOHCperidinineOHOH+OBrEtOBnTfBnNN Al NMe Tf10 mol %OMeO94% ee91:9 drBnOC 9 H 19 MgBrCuBr (10 mol%)THF-78 ºCMeHCCO 2 HC 9 H 19OBn1) AgNO 32) H 2 , Pd/CMeOO94% eeC 9 H 19OH(-)-malyngolideantibioticWan, Z.; Nelson, S. G. J. Am. Chem. Soc. 2000, 122, 10470-10471.
HalogenationsSyn-halogenationMe t -BuHO1H48% aq HBrCuBr, NH 4 BrCu powder"HCuCl 2 "Me t -BuH 2 OLCuBrLSynt -BuMeCHBrOriginal proposed mechanismNo 2 H-exchange at C-1 in D 2 O/DBr,or in H 2 O/HBr with 1-d alcoholLandor, S. R.; Demetriou, B.; Evans, R. J.; Grzeskowiak, R.; Davey, P. J. Chem. Soc., Perkin Trans. 2 1972, 1995-1998.
HalogenationsSyn-halogenationMe t -BuHO1H48% aq HBrCuBr, NH 4 BrCu powder"HCuCl 2 "Me t -BuHOCu BrBrHSynt -BuMeCHBrNo 2 H-exchange at C-1 in D 2 O/DBr,or in H 2 O/HBr with 1-d alcoholLandor, S. R.; Demetriou, B.; Evans, R. J.; Grzeskowiak, R.; Davey, P. J. Chem. Soc., Perkin Trans. 2 1972, 1995-1998.Syn or Anti-halogenation?ClHCHCuCl 2PhHHHPhH~4% eeHCuI 2CantiPhI syn HO~6% eeoptically HCuBrBr2 HpureCPhH~22% eeAuthors conclude syn-elimination is not general for HCuX 2.Substituting for sulfinate ester gave better anti selectivity for X = Cl (24% ee) and X = Br (52% ee). X = I also gave anti, butlow selectivity (~6% ee).Elsevier, C. J.; Vermeer, P. J. Org. Chem. 1984, 49, 1649-1650.
HalogenationsAnti-halogenationPh H SOCl 2HO81% eeEt 2 O0 ºC to rt67%PhHCl18% eeBu 4 NCuCl 2Acetone62%ClHCPhH[α] 25 D -94º (c 0.9, CHCl 3 )Muscio, O. J.; Jun, Y. M.; Philip, Jr., J. B. Tetrahedron Lett. 1978, 2379-2382.H PhHOa) BuLi, THF-70 ºCb) MsClPh H c) cuprate-70 to 20 ºCMsOPhHCXH50% ee[α] 20 D deg (c 2, EtOH)Elsevier, C. J.; Meijer, J.; Tadema, G.; Stehouwer, P. M.; Bos, H. J. T.;Vermeer, P. J. Org. Chem. 1982, 47, 2194-2196.cuprate equiv. X = Cl X = Br X = ILiCuX 2 1.2LiCu 2 X 30.6LiCu 2 X 31.253060061092010401235122013501550
ONBocCHO1) TMSCCLiHMPA, THF, -78 ºC2) TsCl, TEA, DMAPDCM, 0 ºC to rt88%1) TMSCCLiHMPA, THF, -78 ºC2) TsCl, TEA, DMAPDCM, 0 ºC to rt89%HalogenationsOONBocNBocOTsOTsTMSTMSCuBrLiBrTHF60 ºC70%CuBrLiBrTHF60 ºC75%OOHNBocHNBocCCBrTMSTMSBrD'Aniello, F.; Schoenfelder, A.; Mann, A.; Taddei, M. J. Org. Chem. 1996, 61, 9631-9636.HOH1) t-BuOOH(+)-tartrate, 94%1) LDA, THF, 87%TESOEtLiCuBr 267%OHTESOHOEt2) PPh 3 , NCS84%HO HCrimmins, M. T.; Emmitte, K. A. J. Am. Chem. Soc. 2001, 123, 1533-1534OBrHCHHOCl1) PPTS, MeOH2) CBr 4 , oct 3 P58%2) ArSO 2 Cl, 96%ArO 2 SO>98:2 drHO HHBrO H CEtBr H(-)-isolaurallene
RearrangementsMe94% eet-BuOHSOBr 2propyleneoxideEt 2 O, 0 ºCMeBrSOt-BuOMeBrCH+ Met-Bu52% yield, 90:10t-BuBrCoery, E. J.; Boaz, N. W. Tetrahedron Lett. 1984, 25, 3055-3058.Initial studies: Evans, R. J. D.; Landor, S. R.; Smith, R. T. J. Chem. Soc. 1963, 1506-1511.Evans, R. J. D.; Landor, S. R. J. Chem. Soc. 1965, 2553-2559.Ortho Claisen rearrangementOH EtC(OEt) 3t-BuEtCO 2 H98% eeHHCt-BuEtO 2 CMeAMeMeEtOHEOt-BuOHEtO t-BuZ'MeOHEtOt-BuE'MeEtOHrapid equilibrium, E reacts fasterOt-BuZEtO 2 CHMeCBHt-BuA:B = 9:1complete chirality transferHenderson, M. A.; Heathcock, C. H. J. Org. Chem. 1988, 53, 4736-4745.
RearrangementsTMS+TMSOEtSCHOMeSn(OTf)2L* (cat)EtSOMeOTMS92% eeTMS1) deprotection2) MeC(OEt) 3EtCO 2 H86%EtSOCMeTMS92% eeCO 2 EtMukaiyama, T.; Furuya, M.; Ohtsubo, A.; Kobayashi, S. Chem. Lett. 1991, 989-992.TBSOTHPOHOHOTHPOPhMeC(OEt) 3HCO 2 H70%HCO 2 EtCH1 isomerOHOCOHHOPhCO 2 Meenprostil1) CBr 4 , PPh 32)CuCO 2 Me78%HCH1 isomerCO 2 MeCooper, G. F.; Wren, D. L.; Jackson, D. Y.; Beard, C. C.; Galeazzi, E.; Van Horn, A. R.; Li, T. T. J. Org. Chem. 1993, 58, 4280-4286.
RearrangementsOHOTIPS(CH 2 ) 6 CH 3MeC(OEt) 3H +71%EtO 2 CHCHOTIPS(CH 2 ) 6 CH 3ROMeHNMe5Ha, J. D.; Lee, D.; Cha, J. K. J. Org. Chem. 1997, 62, 4550-4551.Ha, J. D.; Cha, J. K. J. Am. Chem. Soc. 1999, 121, 10012-10020.clavepictine A, R = Acclavepictine B, R = H[2,3]-Wittig RearrangementC 7 H 15HO 2 COMe1) LDA2) CH 2 N 280%1) CH 2 N 22) LDA, Cp 2 ZrCl 2THF, -78 ºC, 57%OHMeO 2 C*MeCHC 7 H 15dr 93:7complete transfer<strong>of</strong> chiralityOnediastereomerAgNO 3C 7 H 15MeO 2 COMeOOMOMeHHOMeHMeHFavoredMarshall, J. A.; Robinson, E. D.; Zapata, A. J. Org. Chem. 1989, 54, 5854-5855.Marshall, J. A.; Wang, X.-J. J. Org. Chem. 1990, 55, 2995-2996.Marshall, J. A.; Wang, X.-J. J. Org. Chem. 1990, 56, 4913-4918.MeMeOOMMe
RearrangementsR 1OHR 2NBSHPPh 3 , DEAD-15 ºCHHNNSO 2 ArR 2- HSO 2 ArNH N- N 2HR 1R 2C23 ºC HR 171-91%completetransfer <strong>of</strong>chiralityMeyers, A. G.; Zheng, B. J. Am. Chem. Soc. 1996, 118, 4492-4493.For a synthetic application <strong>of</strong> this, see: Shepard, M. S.Carreira, E. M. J. Am. Chem. Soc. 1997, 119, 2597-2605.R 2ONOHBocClP(OEt) 2TEADCM-10 ºC to rtONHBocCHP(O)(OEt) 2HO 2 CNH 2AP5NMDA antagonistPO 3 H 2Muller, M.; Mann, A.; Taddei, M. Tetrahedron Lett. 1993, 34, 3289-3290.
Palladium CatalysistransmetallationPhXH28% eeX = OAc, OTFA,OS(O)Me2.5-3.2 mol%Pd(PPh 3 ) 4PhZnClTHF/Et 2 O-50 to 25 ºCElsevier, C. J.; Stehouwer, P. M.; Westmijze, H.; Vermeer, P. J. Am. Chem. Soc. 1983, 48, 1103-1105.HPhCL 2 XPdHPhPdXL 2HPhZnClHPhCPhPdL 2HPhHCPhH82:18 anti/syn(Determined bycorrelation <strong>of</strong> rotations)PhBuC85% eeHMeCuI, ZnCl 2i-Pr 2 NH, Pd(PPh 3 ) 4PhTHFBuMe H THFCPhcat. Pd(PPh 3 ) 4OCO 2 Et PhZnBr Bu93% ee 83% eeSubstituting Ph at alkyne terminus resulting in lower stereoselectivityHMeDixneuf, P. H.; Guyot, T.; Ness, M. D.; Roberts, S. M. J. Chem. Soc., Chem. Comm. 1997, 2083-2084.
PhHC10% eeHconditionsX = OHYoshida, M.; Gotou, T.; Ihara, M. Tetrahedron Lett. 2004, 45, 5573-5575.Palladium CatalysistransmetallationPhXH92% ee+B(OH) 210 mol%Pd(PPh 3 ) 4dioxane100 ºC10 min76%X = OCO 2 MePhCH92% eeHO92% eePh+B(OH) 210 mol%Pd(PPh 3 ) 4OHPh2:1 dioxane/H 2 OC80 ºC, 1-3 h92% 92% eeYoshida, M.; Ueda, H.; Ihara, M. Tetrahedron Lett. 2005, 46, 6705-6708.RHCHPh>95% eeconditionsX = OBzR = MeXRH100% ee+Ph 3 InRiveiros, R.; Rodriguez, D.; Sestelo, J. P.; Sarandeses, L. A. Org. Lett. 2006, 8, 1403-1406.Pd(DPEphos)Cl 2THF, rt, 8h70%X = OAc, OBzR = PhHRCHPh84-86% ee
Palladium CatalysisCarbonylationR 11 mol% PdO2 (dba) 3 ⋅CHCl 38 mol% PPhR 4 3OR 1R 1CO (1-30 atm)R 3R 3R 1CR 3OCRL 2 Pd2R 2CORO2R 4 OH, 20-100 ºCR 2 R3OR 4PdL 21-44 hR 4 OR 4O(±) (±)Tsuji, J.; Sugiura, T.; Minami, I. Tetrahedron Lett. 1986, 27, 731-734.44-99% yieldMeO 2 COC 6 H 1370% eeOMOM35 mol% Pd 2 (dba) 320 mol% PPh 3CO (1 atm)2:1 PhH/MeOH50-60 ºC, 4.5 hC 6 H 13HMOMOC70% yield
Palladium CatalysisCarbonylationR 11 mol% PdO2 (dba) 3 ⋅CHCl 38 mol% PPhR 4 3OR 1R 1CO (1-30 atm)R 3R 3R 1CR 3OCRL 2 Pd2R 2CORO2R 4 OH, 20-100 ºCR 2 R3OR 4PdL 21-44 hR 4 OR 4O(±) (±)Tsuji, J.; Sugiura, T.; Minami, I. Tetrahedron Lett. 1986, 27, 731-734.44-99% yieldMsOR 15 examples95% eeR 2Pd(PPh 3 ) 4CO (200 psi)THF, R 3 OHrtR 1HCR 2CO 2 R 380-86% yield80-93% eeIBrCH 2 Cl 2-78 ºC1 hR 1IOR 289-97% yield80-93% eeOMarshall, J. A.; Wolf, M. A. J. Org. Chem. 1996, 61, 3238-3239.Marshall, J. A.; Wolf, M. A.; Wallace, E. M. J. Org. Chem. 1997, 62, 367-371.Pd-catalyzed formation <strong>of</strong> butenolides from allenic acids with high chirality transfer: Ma, S.; Shi, Z. J. Chem. Soc., Chem. Commun. 2002, 540-541.
Palladium CatalysisCarbonylationOH$$14 stepsOOBuLiTHFpentane-78 ºC88%HOOMsClEt 3 NCH 2 Cl 2-78 ºCMsOOPd(PPh 3 ) 4CO (1 atm)TMSCH 2 CH 2 OHTHF75%(2 steps)HOCCO 2 R1 diastereomerR = OCH 2 CH 2 TMSPPh 3CH 3 CN55 ºC92 hHMarshall, J. A.; Wallace, E. M.; Coan, P. S. J. Org. Chem. 1995, 60, 796-797.Marshall, J. A.; Bartley, G. S.; Wallace, E. M. J. Org. Chem. 1996, 61, 5729-5735.COCO 2 R85:151) TBAF, THF93%2) AgNO 3Acetone, 68%OOO(-)-kallolide B(unnatural)
Palladium CatalysisROSOTol87% eeR = Me, Pentyl5 mol% Pd(OAc) 27.5 mol% dppeTHFROOHS PdL 2TolinsertionHRCHPdL 2TolO 2 SreductiveeliminationHRCHSO 2 TolHiroi, K.; Kato, F. Tetrahedron 2001, 57, 1543-1550.2 mol% Pd(acac) 2Ph 2 SiOSiMe 2 PhC 6 H 13Me97% eeNC8 mol%Toluene, refluxCHOMeOSiPh 2C 6 H 13SiMe 2 Phthen BuLiTHF, -78 ºC86%HMeCSiMe 2 PhC 6 H 13TiCl 46 1376% yield95:5 syn:antiMe93% eeSuginome, M.; Matsumoto, A.; Ito, Y. J. Org. Chem. 1996, 61, 4884-4885.
Allenylmetal Compounds1.5 equiv Ti(Oi-Pr) 4TMS3 equiv i-PrMgClR 2 Et 2 O, -50 to -40 ºCR 1AXX = OCO 2 Et or OPO(OEt) 2(i-PrO) 2 XTiTMSCR 2R 1PhCHOTMSPhR 2 R 1OH72-87% yieldTi(Oi-Pr) 4+2 i-PrMgClATi(Oi-Pr)R 2 Ti(Oi-Pr) 22 R 1Ti(Oi-Pr) 2 TMSXTMSR 2 R 1Ph OHPhCHOTi(Oi-Pr) 2CXTi(Oi-Pr) 2R 2 TMSTMSR 1R 1 R 2Okamoto, S.; An, D. K.; Sato, F. Tetrahedron Lett. 1998, 39, 4551-4554.(Mechanism) Nakagawa, T.; Kasatkin, A.; Sato, F. Tetrahedron Lett. 1995, 36, 3207-3210.
Allenylmetal CompoundsTMS97% eeBuHOCO 2 EtTMS(i-PrO) 2 XTiCBuHTMSPh2BuH1OH%ee / Config.49 / (1R,2S)49 / (1S,2S)TMS97% eeBuHOPO(OEt) 2(i-PrO) 2 XTiTMSCBuHTMSPh21OHBuH94 / (1S,2R)94 / (1R,2R)TMS87% eeMeOCO 2 EtTMS(i-PrO) 2 XTiCRMeTMSPh21MeOH85 / (2S)TMS87% eeMeOPO(OEt) 2(i-PrO) 2 XTiTMSCRMeTMSPh21MeOH8.3 / (2R)Okamoto, S.; An, D. K.; Sato, F. Tetrahedron Lett. 1998, 39, 4551-4554.(Mechanism) Nakagawa, T.; Kasatkin, A.; Sato, F. Tetrahedron Lett. 1995, 36, 3207-3210.
Allenylmetal CompoundsOi-PrOi-Pri-PrO TiTMSOBuHPO(OEt) 2anti(i-PrO) 2 XTiTMS2º phosphate(high selectivity)CBuHi-PrO TiTMSOantiMe(OEt) 2PO(i-PrO) 2 XTiTMS3º phosphate(low selectivity)CBuHTMSi-PrOi-PrO OOEtTiOMesynTMS(i-PrO) 2 XTiCRMe2º or 3º carbonate(moderate selectivity)TMS1.5 equiv Ti(Oi-Pr) 43 equiv i-PrMgClR 1 Et 2 O, -50 to -40 ºCHR 2CO 2 EtCO 2 EtTMSCO 2 Et95 or 98% eeOPO(OEt) 2R 1 R 2 CO 2 EtSong, Y.; Okamoto, S.; Sato, F. Org. Lett. 2001, 3, 3543-3545.> 97:3 anti:syn> 88 to 96% eeFor reviews <strong>of</strong> the synthesis and reactivity <strong>of</strong> allenylmetal compounds, see: Marshall, J. A. Chem. Rev. 1996, 96, 31-47. Marshall, J. A. Chem. Rev. 2000,100, 3163-3185. Marshall, J. A.; Gung, B. W.; Grachan, M. L. <strong>Synthesis</strong> and reactions <strong>of</strong> allenylmetal compounds. In Modern Allene Chemistry; Krause, N.,Hashmi, A. S. K., Eds.; Wiley-VCH: Weinheim, 2004; 493-592.
Allylic Elimination (β-elimination)A) <strong>Chiral</strong>ity transfer from allylic positionZLGR 3R 1 R 2X"Activator"XZLGR 3R 1 R 2R 1HCR 2 R 3B) <strong>Chiral</strong> leaving groupsH R H SYoxidationR 4R 3 CR 1 R 2R 1 R 2Y = S, SeR 4OH RY H SR 3- HOYR 4R 1HR 2 R 3
Allylic Elimination (β-elimination)<strong>Chiral</strong>ity at allylic positionMeOHC 6 H 13Bu 3 SnHAIBNneat90 ºCMeOHSnBu 3C 6 H 13SwernMeOSnBu 3C 6 H 13CBSreductionMeOHSnBu 3C 6 H 1394% eeMsClTEACH 2 Cl 20 ºC to rtMeKonoike, T.; Araki, Y. Tetrahedron Lett. 1992, 33, 5093-5096.HCHC 6 H 1351% eeanti elimination
Allylic Elimination (β-elimination)<strong>Chiral</strong>ity at allylic positionMeOHC 6 H 13Bu 3 SnHAIBNneat90 ºCMeOHSnBu 3C 6 H 13SwernMeOSnBu 3C 6 H 13CBSreductionMeOHSnBu 3C 6 H 1394% eeAc 2 ODMAP81%MeKonoike, T.; Araki, Y. Tetrahedron Lett. 1992, 33, 5093-5096.FOAcSnBu 3C 6 H 13TBAFHHCMeC 6 H 1394±2% eeanti eliminationTolOS(R)1)C 8 H 17OHCCO 2 MeLDA, THF, -78 ºC31% (78% brsm)2) Ac 2 OSatoh, T.; Hanaki, N.; Kuramochi, Y.; Inoue, Y.;Hosoya, K.; Sakai, K. Tetrahedron 2002, 58, 2533-2549.51%44%TolTolOSOSROAcC 8 H 17ROAcC 8 H 176 equivi-PrMgCl-78 ºC10 min95%6 equivi-PrMgCl-78 ºC10 min91%HC 8 H 17HC 8 H 17HC81% eeCH84% eeCO 2 MeCO 2 Me
Allylic Elimination (β-elimination)<strong>Chiral</strong>ity at allylic positionOR 1 PPh 21)Ph N PhLiTMSCl, THF, -78 ºC2) H 2 O3) TBAF, THFR 1 R 2OPPh 2OHNaHDMF60 ºCR 1R 2Ph PhPOOR 2 HCR 1HR 1 =Ph, R 2 =t-Bu, 87%, 28% eeR 1 =t-Bu, R 2 =t-Bu, 58%, 51% eeR 1 =Ph, R 2 =t-Bu, 89%, ~12% eeR 1 =t-Bu, R 2 =t-Bu, 74%, ~23% eeThis work: Fox, D. J.; Medlock, J. A.; Vosser, R.; Warren, S. J. Chem. Soc., Perkin Trans. 1 2001, 2240-2249.For related achiral systems, see: Nagaoka, Y.; Tomioka, K. J. Org. Chem. 1998, 63, 6428-6429. Inoue, H.; Tsubouchi, H.; Nagaoka, Y.; Tomioka, K.Tetrahedron 2002, 58, 83-90.
Allylic Elimination (β-elimination)<strong>Chiral</strong> leaving groupsRArSeTsHEt 3 NorKOtBuRArSeTsSAEorDavisEpoxidationnC 7 H 15HOSeArTsNot isolatedCan racemize with H 2 O- ArSeOHnC 7 H 15HC45 examples9-100% yield1-42% eeHTsKomatsu, N.; Murakami, T.; Nishibayashi, Y.; Sugita, T.; Uemura, S. J. Org. Chem. 1993, 58, 3697-3702.
Allylic Elimination (β-elimination)<strong>Chiral</strong> leaving groupsRArSeTsHEt 3 NorKOtBuRArSeTsSAEorDavisEpoxidationnC 7 H 15HOSeArTsNot isolatedCan racemize with H 2 O- ArSeOHnC 7 H 15HC45 examples9-100% yield1-42% eeHTsKomatsu, N.; Murakami, T.; Nishibayashi, Y.; Sugita, T.; Uemura, S. J. Org. Chem. 1993, 58, 3697-3702.Positioned away fromsidechain <strong>of</strong> Fc*RCO 2 EtR = Me, Et, Pr(FcSe) 2NaBH 4EtOH- HOSeFc*RHRSeCFc*HCO 2 EtCO 2 Et38-59% yield75-89% eemCPBACH 2 Cl 24Å MS-78 to -20 ºCRHHCO 2 EtO SeFc*Not isolated(FcSe) 2 =FeMe 2 NSe SeMeFeHishibayashi, Y.; Singh, J. D.; Fukuzawa, S.; Uemura, S. J. Org. Chem. 1995, 60, 4114-4120.MeNMe 2(S,R)
Allylic Elimination (β-elimination)Equilibration <strong>of</strong> allylic metal centerHOSTolBuCu⋅MgBr 2THF-70 to 20 ºCBuHCuS OTolthen, Bu 2 Zn, 2 MgBr 20 to 5 ºCthen,BuIIBuHBuTolSZnBuO"Racemic" sp 3organometallicBuHBuTolSZnBuO<strong>The</strong>rmodynmicequilibration;"Enantioenriched"sp 3 organometallicsynBuHC75% yield65% eeBuHVarghese, J. P.; Zouev, I.; Aufauvre, L.; Knochel, P.; Marek, I. Eur. J. Org. Chem. 2002, 4151-4158.
Stoichiometric <strong>Chiral</strong> Reagents and AuxilliariesA) Asymmetric Deprotonation-ProtonationH R H S B*R 2R 1 R 2B*H SR 1H RH SR 1CR 2EEB) Asymmetric Horner-Wadsworth-EmmonsOCR 1 R 2(*RO) 2OPOORONu C Nu CR 1 R 2R 2R 1HCO 2 R
Asymmetric DeprotonationCbOH R H SBuLi(-)-sparteineR 1R 1 = TMS, t-Bupentane-78 ºCNONOLiNR 1TiCl(Oi-Pr) 3INVERSION!CbOTi(Oi-Pr) 3R 1Precipitates from reactionEquilibrates in THFCbOTi(Oi-Pr) 3R 1AcOHR 1HCOCbHTMS: 86% yield, 88% eet-Bu: 80% yield, 84% eeR 2 CHO R 1 HHR 2OCbTiX 3OR 2R 1 OCbCHOH1 diastereomer73-86% yield93->95% eeThis Work: Schultz-Fademrecht, C.; Wibbeling, B.; Fröhlich, R.; Hoppe, D. Org. Lett. 2001, 3, 1221-1224.For inversion in Li-Ti exchange with allyl species, see: Paulsen, H.; Graeve, C.; Hoppe, D. <strong>Synthesis</strong>, 1996, 141-144.
Asymmetric DeprotonationOORBuLi (2 equiv)THF, -78 ºC, 2 hOOLiHHORt-BuOHOOHCRHR = Me, 55%, 20/1 drR = t-Bu, 65%, 3/1 drBuLiTHF-78 ºC30 minHMePhOOLiCt-BuONOHCl(CF 3 ) 2 CHOHCF 3 CH 2 OHt-BuHCOδPhHδOOHMet-BuHPhOHOOHMeO+t-BuHOOHHt-BuHOOHPhMePhMeThis Work: Harrington, P. E.; Murai, T.; Chu, C.; Tius, M. A. J. Am. Chem. Soc. 2002, 124, 10091-10100.For examples with a fructose-derived auxilliary, see: Hausherr, A.; Orschel, B.; Scherer, S.; Reissig, H.-U. <strong>Synthesis</strong> 2001, 1377-1385.
Asymmetric ProtonationHO+CO 2 Me1) (R)-BINOL-TiCH 2 Cl 2 , 95%2) BuLi(EtO) 2 POCl94%OPO(OEt) 2CO 2 MePd 05 mol% Pd(PPh 3 ) 4SmI 2 (2 equiv)t-BuOH (1.1 equiv)THF53%CRacemic!CO 2 MeR 2Pd IIR 1 CR 2HPd IIR 1 2 Sm IIPd 0HR 1CR 2Pd IIHCR 2Sm IIISm IIIR 1 R*OH R*OHR 1 R 2HR 1CR 2Sm IIIR 1HCR 2HHR 1CR 2HMikami, K.; Yoshida, A. Angew. Chem. Int. Ed. 1997, 36, 858-860.Mikami, K.; Yoshida, A. Tetrahedron 2001, 57, 889-898.
Asymmetric ProtonationHO+CO 2 Me1) (R)-BINOL-TiCH 2 Cl 2 , 95%2) BuLi(EtO) 2 POCl94%OPO(OEt) 2CO 2 Me5 mol% Pd(PPh 3 ) 4SmI 2 (2 equiv)t-BuOH (1.1 equiv)THF53%CRacemic!CO 2 MeOO OH1.1 equiv+racemicOPO(OEt) 2CO 2 Me5 mol% Pd(PPh 3 ) 4SmI 2 (2 equiv)THF68%H>95% eeCHCO 2 MeR'R'HOR'HSmEOHRHOR'RSmEOHHwithPhHOPhOH71% yield86% eeMikami, K.; Yoshida, A. Angew. Chem. Int. Ed. 1997, 36, 858-860.Mikami, K.; Yoshida, A. Tetrahedron 2001, 57, 889-898.
Asymmetric HWER 1R 2CO 2 BHTBuLiZnCl 2THF-78 ºCR 1R 2COKHMDSATHF-78 ºC to rtR 1R 2CH60-94% yield4-84% eeCO 2 MeR 1R 2CO 2 PhLDAZnCl 2THF-78 ºCR 1R 2COLDAATHF-78 ºC to rtR 1R 2CH21-71% yield32-89% eeCO 2 MeMesi faceOCOPOMeAOOOMeMeOOOPMeZn 2+OSOMeLre faceblocked by MeThis work: Tanaka, K.; Otsubo, K.; Fuji, K. Tetrahedron Lett. 1996, 37, 3735-3738. Yamazaki, J.; Watanabe, T.; Tanaka, K. Tetrahedron: Asymmetry 2001, 12,669-675.For examples <strong>of</strong> Wittig-type olefinations <strong>of</strong> ketenes, see: Lang, R. W.; Hansen, H. J. Helv. Chim. Acta 1980, 63, 438-55. Tanaka, K.; Otsubo, K.; Fuji, K. Synlett1995, 933-934. Tanaka, K.; Otsubo, K.; Fuji, K. Tetrahedron Lett. 1995, 36, 9513-9514.
<strong>Chiral</strong> Catalysis and Kinetic resolution
Kinetic ResolutionHC 7 H 15CHOHSharplessAsymmetricEpoxidationrun to ~60% conversionC 7 H 15HCHOH40% eeSharpless, K. B.; Behrens, C. H.; Katsuki, T.; Lee, A. W. M.; Martin, V. S.; Takatani, M.; Viti, S. M.; Walker, F. J.; Woodard, S. S. Pure Appl. Chem. 1983, 55,589-604.RCH2 mol% catPhIORCHHRCH 2 Cl 2-40 ºCHRNoguchi, Y.; Takiyama, H.; Katsuki, T. Synlett 1998, 543-545Me Me61-98% ee57-83% conversionMeMeN NMn +O OPh Ph
HKinetic Resolution(S,S)ZrNArRC R1.8 equivHC 6 H 6RHCRH(R)50-61% conv78-98% ee+ZrHArNR(S,S,R)HRCHHCRR(S)>95% recovery85-93% eeZrNArC1 equivZrHArNH(S,S)100% conv.(S,S,R)1 diastereomer!ZrNRArCHRThought to involve stepwisemechanism; therefore highlystereoselective, not stereospecificCHOnly favoredapproachHC(S)H84% ee93% yieldSweeney, Z. K.; Salsman, J. L.; Anderson, R. A.; Bergman, R. G. Angew. Chem. Int. Ed. 2000, 39, 2339-2343.
Kinetic ResolutionderacemizationRO 2 CHCHCO 2 RR = (-)-L-menthyl5:4 dr1 mol% Et 3 Npentane-20 ºC2 daysRO 2 CHCCO 2 RH(R) crystallizes90% yield>98% deNode, M.; Nishide, K.; Fujiwara, T.; Ichihashi, S. J. Chem. Soc., Chem. Commun.1998, 2363-2364.RO 2 CHC(R)CO 2 RH+ Et 3 N- Et 3 NORO 2 CHORHNEt 3- Et 3 N+ Et 3 NRO 2 CHC(S)HCO 2 RRO 2 CHBocNCO 2 R AlClC3HH+CH 2 Cl 2C-78 ºC HN BocCO 2 RCO 2 RBocNHH CO 2RCO 2 RHNH(-)-epibatidineNCl
Kinetic ResolutionderacemizationRO 2 CHCHCO 2 RR = (-)-L-menthyl5:4 dr1 mol% Et 3 Npentane-20 ºC2 daysRO 2 CHCCO 2 RH(R) crystallizes90% yield>98% deNode, M.; Nishide, K.; Fujiwara, T.; Ichihashi, S. J. Chem. Soc., Chem. Commun.1998, 2363-2364.i-PrO 2 CHCHCO 2 i-Pr(+)-Eu(hfc) 3CDCl 39 days20 ºCi-PrO 2 CHC(S)>95% eeHCO 2 i-PrNaruse, Y.; Watanabe, H.; Ishiyama, Y.; Yoshida, T. J. Org. Chem. 1997, 62, 3862-3866
<strong>Chiral</strong> Catalysis<strong>The</strong> Beginningt-BuHCHHBuLiMXTHF-60 ºCt-BuMX = ZnCl, MgCl, CuBr/LiBrHC(±)HMcat Pd(R,R-DIOP)PhI-60 ºC to rtt-BuHCup to 25% eechanging MX altered sense <strong>of</strong> inductionHPh"fast" reactingenantiomert-BuHCHM-M + t-BuCHHt-BuHWarning!t-Bu-MM+ t-Bu"slow" reactingCCenantiomer SpeculationH HHby AMH HH t-BuPhIL 2 Pd 0t-BuHIL 2 PdPhCHMMIt-BuHCH PdL 2Ph-L 2 Pd 0t-BuHCHPhde Graaf, W.; Boersma, J.; van Koten, G.; Elsevier, C. J. J. Organometallic Chem. 1989, 378, 115-124.
R 1Br+EtO 2 CMeCO 2 Et<strong>Chiral</strong> CatalysisEtO 2 CNHAcCO 2 EtOgasawara, M.; Ikeda, H.; Nagano, T.; Hayashi, T. J. Am. Chem. Soc. 2001, 123, 2089-2090.Ogasawara, M.; Ikeda, H.; Nagano, T.; Hayashi, T. J. Org. Chem. 2005, 70, 5764-5767.or10 mol%Pd[(R)-BINAP] 2dba (2 equiv to Pd)CsOt-BuCH 2 Cl 2 , 20 ºCHR 1CH34-75% yield54-89% eeCO 2 EtR 2CO 2 Et22R 1BrPd 0 (BINAP)BrR 1 PdPdP P(2R) P PR 134:66 drby NMRR 1(2S)PPdPNuNu<strong>The</strong> presence <strong>of</strong> dbadoes not affect the ratio <strong>of</strong>2R to 2S, but accelerates theinterconversion 12-25 times.HR 1CmajorHNuHR 1CminorHNu
R 1Br+EtO 2 CMeCO 2 Et<strong>Chiral</strong> CatalysisEtO 2 CNHAcCO 2 EtOgasawara, M.; Ikeda, H.; Nagano, T.; Hayashi, T. J. Am. Chem. Soc. 2001, 123, 2089-2090.Ogasawara, M.; Ikeda, H.; Nagano, T.; Hayashi, T. J. Org. Chem. 2005, 70, 5764-5767.or10 mol%Pd[(R)-BINAP] 2dba (2 equiv to Pd)CsOt-BuCH 2 Cl 2 , 20 ºCHR 1CH34-75% yield54-89% eeCO 2 EtR 2CO 2 EtTMSHCH87% eeCO 2 EtNHAcCO 2 EtOgasawara, M.; Ueyama, K.; Nagano, T.; Mizuhata, Y.; Hayashi, T.Org. Lett. 2003, 5, 217-219.TMSHt-BuOMeCFavoredHHR+(S)OMeOMeTMSTMSTiCl 4CH 2 Cl 2-78 ºCHHHt-BuCt-BuMeOt-BuMeOCMeODisfavoredHH(S)HHRRCO 2 EtNHAcCO 2 Et90% yield85% ee(R)
R 1Br+EtO 2 CMeCO 2 Et<strong>Chiral</strong> CatalysisEtO 2 CNHAcCO 2 EtOgasawara, M.; Ikeda, H.; Nagano, T.; Hayashi, T. J. Am. Chem. Soc. 2001, 123, 2089-2090.Ogasawara, M.; Ikeda, H.; Nagano, T.; Hayashi, T. J. Org. Chem. 2005, 70, 5764-5767.or10 mol%Pd[(R)-BINAP] 2dba (2 equiv to Pd)CsOt-BuCH 2 Cl 2 , 20 ºCHR 1CH34-75% yield54-89% eeCO 2 EtR 2CO 2 EtTMSHCH87% eeCO 2 EtNHAcCO 2 EtOgasawara, M.; Ueyama, K.; Nagano, T.; Mizuhata, Y.; Hayashi, T.Org. Lett. 2003, 5, 217-219.+OMeOMeTiCl 4CH 2 Cl 2-78 ºCt-BuMeOH(S)CO 2 EtNHAcCO 2 Et90% yield85% eeR 1HC(±)HOPO(OEt) 2+R 2 O 2 CR 3CO 2 R 2Imada, Y.; Ueno, K.; Kutsuwa, K.; Murahashi, S.-I. Chem. Lett. 2002, 5, 140-141.1 mol%Pd 2 (dba) 3 ⋅CHCl 34 mol%(R)-MeOBIPHEPBSA, THF, rtHR 1CH72-90% yield69-90% eeCO 2 R 2R 3CO 2 R 2
<strong>Chiral</strong> CatalysisR+PdCl(Allyl) 2 /L(1 mol% Pd)HSiCl 3(neat) 20 ºCRCl 3 SiCHMeMeMgBrEt 2 ORTMSCHMe37-90% yield68-90% eeR+Cl 3 Si Pd HLRCl 3 SiPd HLRCl 3 SiPdMeLRCl 3 SiCHMeMeFeRCl 3 SiCHMePhCHODMFRMeOHPh+R1:1 to 4:1100% chirality transferMeOHPhMeOPPhFeOMeMe(S)-(R)-bisPPFOMeHan, J. W.; Tokunaga, N.; Hayashi, T. J. Am. Chem. Soc. 2001, 123, 12915-12916.For hydrosilylation <strong>of</strong> butadiynes with low selectivity, see: Tillack, A., Koy, C.; Michalix, D.; Fischer, C. J. Organometallic Chem. 2000, 603, 116-121.For hydroboration <strong>of</strong> ene-ynes with low selectivity, see: Matsumoto, Y.; Naito, M.; Uozumi, Y.; Hayashi, T. J. Chem. Soc., Chem. Commun. 1993, 1468-1469.
<strong>Chiral</strong> CatalysisnOR[RhCl(C 4 H 4 ) 2 ] 2 /L*(3 or 10 mol% Rh)ArTi(Oi-Pr) 4 LiTMSClTHF, 20 ºC30 minnOTMSC*RAr61->99% yield26-93% ee(configuration not reported)[Rh]-ArTMSClArTi(Oi-Pr) 4 LiOO[Rh]R[Rh]ArisomerizationdeterminesstereochemistryC*RArL* =OOOPPh 2PPh 2Hayashi, T.; Tokunaga, N.; Inoue, K. Org. Lett. 2004, 6, 305-307.O(R)-segphos
ConclusionsPresented a flavor <strong>of</strong> the methods known to construct axial chiral allenes in an enantioselectivemanner, as well as a few reactions these products are useful for.Many reliable methods for chirality transfer from pre-generated stereocenters withstiochiometric reagents.<strong>The</strong>re is still a deficiency in catalytic asymmetric methods. <strong>The</strong> selectivities <strong>of</strong> these processesare not as reliable, and scope has yet to be defined.<strong>Allenes</strong> are not just theoretical curiosities and can be useful synthons for asymmetric synthesisas well as potentially useful final targets for biologically relavant molecules.Pr<strong>of</strong>. Kay BrummondUniversity <strong>of</strong> PittsburghPr<strong>of</strong>. James MarshallUniversity <strong>of</strong> VirginiaPr<strong>of</strong>. Tamio HayashiKyoto University