Concentration Terms and Calculations - Quantum
Concentration Terms and Calculations - Quantum
Concentration Terms and Calculations - Quantum
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
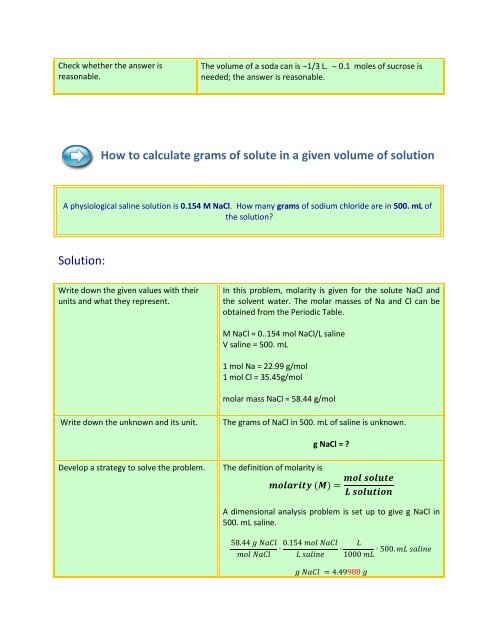
Check whether the answer isreasonable.The volume of a soda can is 1/3 L. 0.1 moles of sucrose isneeded; the answer is reasonable.How to calculate grams of solute in a given volume of solutionA physiological saline solution is 0.154 M NaCl. How many grams of sodium chloride are in 500. mL ofthe solution?Solution:Write down the given values with theirunits <strong>and</strong> what they represent.In this problem, molarity is given for the solute NaCl <strong>and</strong>the solvent water. The molar masses of Na <strong>and</strong> Cl can beobtained from the Periodic Table.M NaCl = 0..154 mol NaCl/L salineV saline = 500. mL1 mol Na = 22.99 g/mol1 mol Cl = 35.45g/molmolar mass NaCl = 58.44 g/molWrite down the unknown <strong>and</strong> its unit.The grams of NaCl in 500. mL of saline is unknown.g NaCl = ?Develop a strategy to solve the problem.The definition of molarity isA dimensional analysis problem is set up to give g NaCl in500. mL saline.