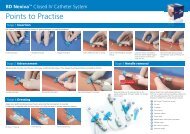
Tubes - BD
Tubes - BD
Tubes - BD
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>BD</strong> Biosciences - Discovery Labware<br />
<strong>BD</strong> Falcon In Vitro Fertilization Plasticware<br />
• Pre‑tested and certified plasticware for in vitro fertilization (IVF)<br />
• Ce marked to the Medical Device Directive 93/42/eC<br />
Features<br />
• Non‑embryotoxic<br />
• Non‑pyrogenic<br />
• Non‑cytotoxic<br />
• Tissue culture‑treated for a consistent hydrophilic surface<br />
• Sterilized by gamma irradiation<br />
• Packaged in peel‑open, medical‑style packaging<br />
• Multi‑unit bags have reseal tabs<br />
Description Qty./Pack Qty./case cat. no.<br />
60 mm Diameter Dish 20 500 353652<br />
well area: 21.29 cm 2<br />
well Volume: 23.0 ml<br />
center-well, 20 500 353653<br />
60 mm Diameter Dish<br />
well area: 2.89 cm 2<br />
well Volume: 2.5 ml<br />
total Volume: 20 ml<br />
4-well Plate 1 100 353654<br />
well area: 1.39 cm 2<br />
well Volume: 1.8 ml<br />
Note: The lids of the <strong>BD</strong> Falcon IVF Products are not certified non-embryotoxic.<br />
<strong>BD</strong> Falcon cell cultureware<br />
Pretested <strong>BD</strong> Falcon IVF products<br />
<strong>BD</strong> Falcon In Vitro Fertilization (IVF) products are the first<br />
plasticware available that is certified sterile, non‑pyrogenic and non‑<br />
embryotoxic. These pre‑tested products save you time and expense<br />
in complying with the College of American Pathologists (CAP) and<br />
American Fertility Society (AFS) recommended standards for IVF labs.<br />
Mouse embryo testing<br />
Products are manufactured in compliance with cGMP standards.<br />
each lot of <strong>BD</strong> Falcon IVF product is tested for embryotoxicity using<br />
the 1‑cell mouse embryo assay. Mouse embryos are isolated at<br />
the 1‑cell stage from B6C3F1 females following super ovulation<br />
with gonadotropin and mating with B6D2F1 males. <strong>BD</strong> Falcon IVF<br />
products are tested using both a direct and indirect test method. In<br />
the direct assay, the embryos are cultured directly in the IVF labware.<br />
For the indirect assay, culture media is incubated in the IVF labware<br />
for 24 hours at 37ºC. The incubated media is then transferred to<br />
a culture plate and the 1‑cell mouse embryo assay is performed.<br />
Products are deemed non‑embryotoxic if they support the growth<br />
of more than 75 percent of the embryos to the expanded and/or<br />
hatched blastocyst stage.<br />
To improve manipulation of ova and embryos, <strong>BD</strong> Biosciences<br />
designed, in conjunction with embryologists, an innovative 4‑well<br />
plate. A unique lid reduces the risk of contamination and minimizes<br />
evaporation by providing access to two wells at a time, while two<br />
remain covered. The wells are numbered and a large writing patch<br />
allows clear sample identification. Plates are packaged in individual<br />
peel‑open trays for sterile presentation.<br />
<strong>BD</strong> Falcon IVF dishes and 4‑well plates are manufactured from virgin<br />
crystalline polystyrene tested for USP Class VI cytotoxicity. They<br />
have flat, optically clear surfaces for optimum manipulation and<br />
observation of ova and embryos. Lids were designed for aseptic<br />
manipulation and consistent venting to maintain humidification.<br />
Individual certificates,<br />
containing actual test<br />
results, are available for<br />
each lot by calling your<br />
local <strong>BD</strong> office after you<br />
receive your order.<br />
4<br />
IVF<br />
bdbiosciences.com | 49