Vitalograph PEF/FEV1 Diary
Vitalograph PEF/FEV1 Diary
Vitalograph PEF/FEV1 Diary
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Vitalograph</strong> <strong>PEF</strong>/FEV 1 <strong>Diary</strong>For the accurate measurement andcollection of <strong>PEF</strong>, FEV 1 and <strong>Diary</strong> datafor home recordingthe difference is clearThe <strong>Vitalograph</strong> <strong>PEF</strong>/FEV 1 <strong>Diary</strong> is ideal for subjects to take home formeasuring and capturing <strong>PEF</strong>, FEV 1 and <strong>Diary</strong> data for remote assessmentand analysis from a wide range of applications including:Clinical TrialsHospitalsOccupational HealthGeneral PracticeThis pocket-size device can be used to assess the effectiveness of newasthma and COPD drugs, asthma management plans and closely monitorrespiratory data from a variety of subjects, including heart-lungtransplant patients.<strong>Vitalograph</strong> is a world leadingprovider of outstanding qualityrespiratory diagnostic devices,clinical trial services and medicalequipment servicing. With apioneering heritage ofexcellence spanning half acentury <strong>Vitalograph</strong> continuesto make valuable contributionsto effective medical care andenhanced quality of life.The compact and lightweight design of the <strong>Vitalograph</strong> <strong>PEF</strong>/FEV 1<strong>Diary</strong> makes it easily portable for use at home or at work and thereplaceable / washable flowhead assures complete hygiene.www.vitalograph.com
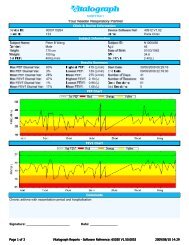
The standard <strong>Vitalograph</strong> <strong>PEF</strong>/<strong>FEV1</strong> <strong>Diary</strong> includes:Entry of symptom scores and medication usage – up to 6 questionsUp to 4 alarm prompts in 24 hours to aid subject compliance6 different types of event markers, such as inhaler usageStorage up to 540 testsTime and date stamping of results with data stored in non-volatile memoryFully programmable set-up for clinical research useIdentification of best test and recording or number of blows/sessionstored against each testTest QA including repeatability and good/poor sessionOptional Microsoft® Windows® based configuration software offering:- allocation of <strong>Diary</strong> to patient with patient specific configuration- downloading stored results to database- trending of results by age or date- event marker- colour zones- variation as % of predicted valuesThe <strong>Vitalograph</strong> <strong>PEF</strong>/FEV 1 <strong>Diary</strong> can be customised for use inrespiratory clinical trials to provide:Integration to clinic based centralized Spirotrac spirometrysystem softwareStorage of all <strong>PEF</strong> and FEV 1 blowsTime windows for recordings, am and pm<strong>Diary</strong> questions with a range of answers, am and pmUse of numeric score with patient instruction cards ensuresconsistency of responseExacerbation alertsCompliance information via Spirotrac® softwareAutomatic daylight saving corrections<strong>Vitalograph</strong> Ltd.Maids MoretonBuckinghamMK18 1SW EnglandTel: +44 (0) 1280 827110Fax: +44 (0) 1280 823302E-mail: pharma@vitalograph.co.uk<strong>Vitalograph</strong> Inc.13310 West 99th StreetLenexaKansas 66215 USATel.: (913) 888 4221Fax: (913) 888 4259E-mail: vitcs@vitalograph.com<strong>Vitalograph</strong> GmbH.Jacobsenweg 1222525 HamburgGermanyTel: (040) 547391-0Fax: (040) 547391-40E-mail: info@vitalograph.de<strong>Vitalograph</strong> (Ireland) Ltd.Gort Road Business ParkEnnisCo. Clare IrelandTel.: (065) 6864100Fax: (065) 6829289E-mail: sales@vitalograph.ieReferences:Peak flow diaries in childhood asthmaare unreliableA W A Kamps, R J Roorda, P L P BrandThorax 2001;56:180–182Evaluation of an electronic hand-heldspirometer in patients with asthmaS.Hamid, Z.M.Corden, D.P.Ryan,I.Burnett, G.M. CochraneRespiratory Medicine (1988) 92,1177-1180Technical Specification:Product: <strong>Vitalograph</strong> <strong>PEF</strong>/<strong>FEV1</strong> <strong>Diary</strong>Model No: 2110Size: 145mm x 70mm x 87mmWeight: 147g (without PP3 battery)Parameters: <strong>PEF</strong>; <strong>FEV1</strong>QA Test Review: Using ATS/ERS 2005Guidelines Operating TemperatureRange: ATS Limits: 17-37°CDesign Limits: 10 - 40°CPower supply:Primary Battery: Alkaline 9V, PP3(MN1604)Flow Detection Principle: Fleisch typepneumotachographVolume Detection: Flow Integrationsampling @ 100HzAccuracy when operated intemperature range: Flow +/- 10% or +/-20 l/min whichever is greaterMax flow rate +/-16L/sMin flow rate +/-0.02L/sVolume Accuracy: +/- 3% or +/- 0.05 lwhichever is greaterMaximum Displayed Volume: 9.99 LitresPerformance Standards: ATS/ERS 2005& EN13826Safety Standards: EN ISO 60601-1:2005-1 -2 -4Designed & Manufactured Under:Medical Devices Directive93/42/EEC1993 ISO 13485:2003, FDA21CFR820, CMDR & JPALCommunications:USB (using converter) and RS232Ordering Info:Catalogue No.20242 SafeTway® Mouthpieces (200)20303 Disposable Noseclips (200)20980 Mini SafeTway® Mouthpieces (50)20991 Long SafeTway® Mouthpieces (130)43104 Plastic Mouthpieces (150)36020 3-L Precision Syringe63053 Flowheads (1)73302 PC Software2110201 Hydrophilic Filters (20)2110203/4 Action Plan Labels 4/3 zone (25)63505 Intelligent printer cable63502 Serial cable for 211065030SPR <strong>Vitalograph</strong> Reports Utility77051 USB to Serial converterwww.vitalograph.com<strong>Vitalograph</strong>® , SafeTway® and Spirotrac® are trademarks of <strong>Vitalograph</strong> Ltd. Microsoft TM is aregistered trademark and Windows TM is a trademark of Microsoft Corporation.PRINT REF: 14503_1