Determination of calcium and magnesium in milk
Determination of calcium and magnesium in milk
Determination of calcium and magnesium in milk
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
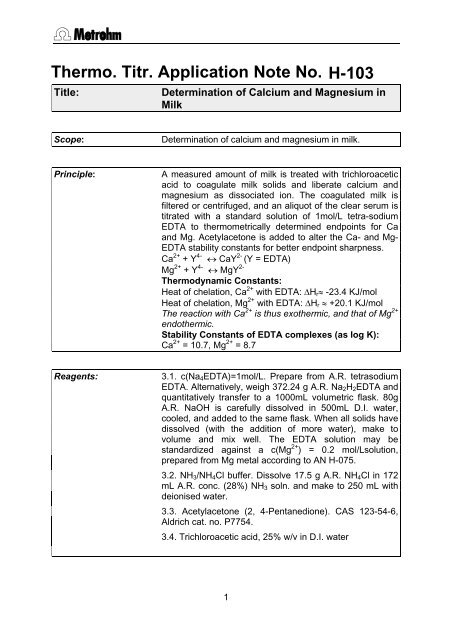
Thermo. Titr. Application Note No.Title:H-103<strong>Determ<strong>in</strong>ation</strong> <strong>of</strong> Calcium <strong>and</strong> Magnesium <strong>in</strong>MilkScope:<strong>Determ<strong>in</strong>ation</strong> <strong>of</strong> <strong>calcium</strong> <strong>and</strong> <strong>magnesium</strong> <strong>in</strong> <strong>milk</strong>.Pr<strong>in</strong>ciple:A measured amount <strong>of</strong> <strong>milk</strong> is treated with trichloroaceticacid to coagulate <strong>milk</strong> solids <strong>and</strong> liberate <strong>calcium</strong> <strong>and</strong><strong>magnesium</strong> as dissociated ion. The coagulated <strong>milk</strong> isfiltered or centrifuged, <strong>and</strong> an aliquot <strong>of</strong> the clear serum istitrated with a st<strong>and</strong>ard solution <strong>of</strong> 1mol/L tetra-sodiumEDTA to thermometrically determ<strong>in</strong>ed endpo<strong>in</strong>ts for Ca<strong>and</strong> Mg. Acetylacetone is added to alter the Ca- <strong>and</strong> Mg-EDTA stability constants for better endpo<strong>in</strong>t sharpness.Ca 2+ + Y 4- CaY 2- (Y = EDTA)Mg 2+ + Y 4- MgY 2-Thermodynamic Constants:Heat <strong>of</strong> chelation, Ca 2+ with EDTA: H r -23.4 KJ/molHeat <strong>of</strong> chelation, Mg 2+ with EDTA: H r +20.1 KJ/molThe reaction with Ca 2+ is thus exothermic, <strong>and</strong> that <strong>of</strong> Mg 2+endothermic.Stability Constants <strong>of</strong> EDTA complexes (as log K):Ca 2+ = 10.7, Mg 2+ = 8.7Reagents:3.1. c(Na 4 EDTA)=1mol/L. Prepare from A.R. tetrasodiumEDTA. Alternatively, weigh 372.24 g A.R. Na 2 H 2 EDTA <strong>and</strong>quantitatively transfer to a 1000mL volumetric flask. 80gA.R. NaOH is carefully dissolved <strong>in</strong> 500mL D.I. water,cooled, <strong>and</strong> added to the same flask. When all solids havedissolved (with the addition <strong>of</strong> more water), make tovolume <strong>and</strong> mix well. The EDTA solution may best<strong>and</strong>ardized aga<strong>in</strong>st a c(Mg 2+ ) = 0.2 mol/Lsolution,prepared from Mg metal accord<strong>in</strong>g to AN H-075.3.2. NH 3 /NH 4 Cl buffer. Dissolve 17.5 g A.R. NH 4 Cl <strong>in</strong> 172mL A.R. conc. (28%) NH 3 soln. <strong>and</strong> make to 250 mL withdeionised water.3.3. Acetylacetone (2, 4-Pentanedione). CAS 123-54-6,Aldrich cat. no. P7754.3.4. Trichloroacetic acid, 25% w/v <strong>in</strong> D.I. water1
Method:Basic Experimental Parameters:Titrant delivery rate (mL/m<strong>in</strong>.) 4ERC Ca EP -15ERC Mg EP +5Data smooth<strong>in</strong>g factor (DSF) 15Stirr<strong>in</strong>g speed (802 stirrer) 15Delay before start <strong>of</strong> titration (secs.) 10Prepare an aliquot <strong>of</strong> serum <strong>of</strong> the <strong>milk</strong> product bypipett<strong>in</strong>g 100 mL <strong>of</strong> <strong>milk</strong> <strong>in</strong>to a 250 mL beaker equippedwith a large magnetic sp<strong>in</strong> bar. Set on a magnetic stirrer,<strong>and</strong> slowly add by bulb pipette 10 mL 25% w/vtrichloroacetic acid soluton. Allow to stir for 10 m<strong>in</strong>utes.Separate clarified <strong>milk</strong> serum either by filtration through aWhatman no. 4 filter paper or by centrifugation. Pipette 50mL <strong>of</strong> <strong>milk</strong> serum <strong>in</strong>to a titration vessel <strong>and</strong> add 800 µLacetylacetone. Prior to the titration with Na 4 EDTA, 5 mL <strong>of</strong>NH 3 /NH 4 Cl buffer is added automatically by Dos<strong>in</strong>o. Thevolume <strong>of</strong> orig<strong>in</strong>al <strong>milk</strong> conta<strong>in</strong>ed <strong>in</strong> a 50 mL aliquotdelivered for titration is 100*50/(100+10)=45.4545 mLFull cream<strong>milk</strong>Low fat <strong>milk</strong>Skim <strong>milk</strong>with <strong>milk</strong>solids <strong>and</strong><strong>milk</strong> <strong>calcium</strong>NutritionInformation onlabelCa mg/100 mLTitrotherm Camg/100 mLTitrotherm Mgmg/100 mL117 145 175125.9±0.50(n=5)13.2±0.41(n=5)147.0±0.44(n=9)14.8±0.59(n=9)209.7±0.45(n=8)14.5±0.33(n=9)Calculations:Ca mg/100 mL =((EP1-Ca blank)*M EDTA*40.078*100)/sample vol., mLMg mg/100 mL = ((EP2-EP1-Mg blank)*M EDTA*24.305*100)/sample vol,mL2
<strong>Determ<strong>in</strong>ation</strong> <strong>of</strong> Ca <strong>and</strong> Mg blanksThe method blank is determ<strong>in</strong>ed by titrat<strong>in</strong>g differentamounts <strong>of</strong> a representative sample <strong>of</strong> the product <strong>and</strong>plott<strong>in</strong>g the sample amount aga<strong>in</strong>st the titrantconsumption. The method blank is determ<strong>in</strong>ed as the y-<strong>in</strong>tercept from a l<strong>in</strong>ear regression <strong>of</strong> the titration data.Changes <strong>in</strong> titrant dose rate or filter factor will require anew determ<strong>in</strong>ation <strong>of</strong> the method blank.In the case <strong>of</strong> this determ<strong>in</strong>ation, it is necessary tocompute two method blanks: one for the Ca endpo<strong>in</strong>t(EP1) <strong>and</strong> one for the Mg endpo<strong>in</strong>t (EP2). There ismutual <strong>in</strong>terference between Ca <strong>and</strong> Mg dur<strong>in</strong>g thetitration, <strong>and</strong> this must be compensated correctly.To obta<strong>in</strong> an accurate estimate for the Mg blank,separate regression analyses are performed on EP1<strong>and</strong> EP2, with the <strong>in</strong>tercept for EP1 be<strong>in</strong>g subtractedfrom that for EP2.Examples <strong>of</strong> Ca <strong>and</strong> Mg blank estimations:Full cream<strong>milk</strong>Low fat <strong>milk</strong>Skim <strong>milk</strong>with <strong>milk</strong>solids <strong>and</strong><strong>milk</strong> <strong>calcium</strong>Ca <strong>in</strong>tercept(blank), mL(1)Mg <strong>in</strong>tercept,mL (2)CorrectedMg blank(2)-(1)0.0623 0.0547 0.03530.1320 0.1329 0.16260.0697 0.0782 0.12743
Examples <strong>of</strong> titration plotsBlue curve = solutiontemperatureBlack curve = secondderivative <strong>of</strong> solutiontemperatureEP1 = <strong>calcium</strong> endpo<strong>in</strong>t(exothermic reaction,negative endpo<strong>in</strong>t“peak”)EP2 = <strong>magnesium</strong>endpo<strong>in</strong>t (endothermicreaction, positiveendpo<strong>in</strong>t “peak”)Fig. 1. Ca <strong>and</strong> Mg <strong>in</strong> full cream <strong>milk</strong>Fig. 2. Ca <strong>and</strong> Mg <strong>in</strong> low fat <strong>milk</strong>Fig. 3. Ca <strong>and</strong> Mg <strong>in</strong> skim <strong>milk</strong> with added <strong>milk</strong> solids <strong>and</strong> <strong>milk</strong> <strong>calcium</strong>Notes on safe usage <strong>and</strong> disposal <strong>of</strong> trichloroacetic acid.CCl 3 COOH (trichloroacetic acid) <strong>and</strong> its solutions are toxic <strong>and</strong> corrosive. Wearappropriate protective cloth<strong>in</strong>g. Adhere to recommendations <strong>in</strong> MSDSdocumentation. Disposal <strong>of</strong> CCl 3 COOH solutions <strong>and</strong> residues conta<strong>in</strong><strong>in</strong>g CCl 3 COOHshould be <strong>in</strong> accordance with local regulations.4






![Rice, size measurement of broken grains [pdf] - MEP Instruments](https://img.yumpu.com/46724497/1/184x260/rice-size-measurement-of-broken-grains-pdf-mep-instruments.jpg?quality=85)