who medicines strategy - libdoc.who.int - World Health Organization
who medicines strategy - libdoc.who.int - World Health Organization
who medicines strategy - libdoc.who.int - World Health Organization
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
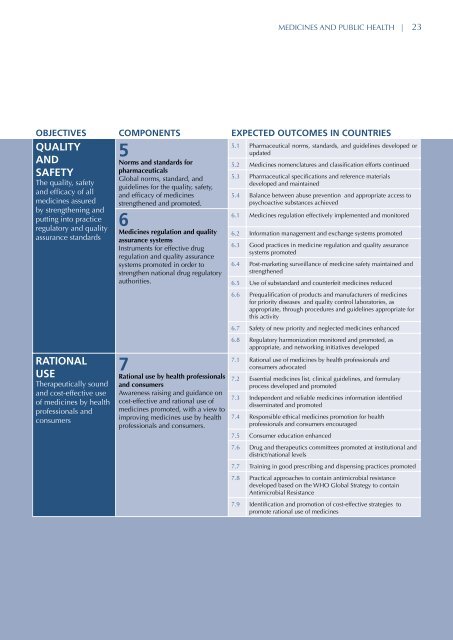
MEDICINES AND PUBLIC HEALTH | 23OBJECTIVES COMPONENTS EXPECTED OUTCOMES IN COUNTRIESQUALITY5ANDNorms and standards forSAFETYpharmaceuticalsGlobal norms, standard, and 5.3 Pharmaceutical specifications and reference materialsThe quality, safetydeveloped and ma<strong>int</strong>ainedguidelines for the quality, safety,and efficacy of alland efficacy of <strong>medicines</strong><strong>medicines</strong> assured strengthened and promoted.by strengthening andputting <strong>int</strong>o practiceregulatory and qualityassurance standardsRATIONALUSETherapeutically soundand cost-effective useof <strong>medicines</strong> by healthprofessionals andconsumers6Medicines regulation and qualityassurance systemsInstruments for effective drugregulation and quality assurancesystems promoted in order tostrengthen national drug regulatoryauthorities.7Rational use by health professionalsand consumersAwareness raising and guidance oncost-effective and rational use of<strong>medicines</strong> promoted, with a view toimproving <strong>medicines</strong> use by healthprofessionals and consumers.5.1 Pharmaceutical norms, standards, and guidelines developed orupdated5.2 Medicines nomenclatures and classification efforts continued5.4 Balance between abuse prevention and appropriate access topsychoactive substances achieved6.1 Medicines regulation effectively implemented and monitored6.2 Information management and exchange systems promoted6.3 Good practices in medicine regulation and quality assurancesystems promoted6.4 Post-marketing surveillance of medicine safety ma<strong>int</strong>ained andstrengthened6.5 Use of substandard and counterfeit <strong>medicines</strong> reduced6.6 Prequalification of products and manufacturers of <strong>medicines</strong>for priority diseases and quality control laboratories, asappropriate, through procedures and guidelines appropriate forthis activity6.7 Safety of new priority and neglected <strong>medicines</strong> enhanced6.8 Regulatory harmonization monitored and promoted, asappropriate, and networking initiatives developed7.1 Rational use of <strong>medicines</strong> by health professionals andconsumers advocated7.2 Essential <strong>medicines</strong> list, clinical guidelines, and formularyprocess developed and promoted7.3 Independent and reliable <strong>medicines</strong> information identifieddisseminated and promoted7.4 Responsible ethical <strong>medicines</strong> promotion for healthprofessionals and consumers encouraged7.5 Consumer education enhanced7.6 Drug and therapeutics committees promoted at institutional anddistrict/national levels7.7 Training in good prescribing and dispensing practices promoted7.8 Practical approaches to contain antimicrobial resistancedeveloped based on the WHO Global Strategy to containAntimicrobial Resistance7.9 Identification and promotion of cost-effective strategies topromote rational use of <strong>medicines</strong>