who medicines strategy - libdoc.who.int - World Health Organization
who medicines strategy - libdoc.who.int - World Health Organization
who medicines strategy - libdoc.who.int - World Health Organization
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
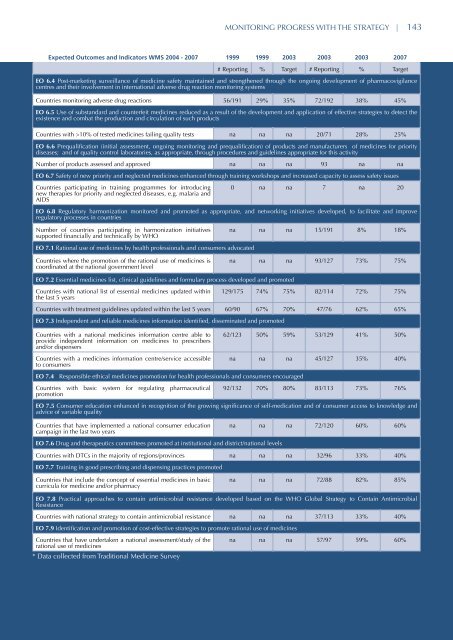
MONITORING PROGRESS WITH THE STRATEGY | 143Expected Outcomes and Indicators WMS 2004 - 2007 1999 1999 2003 2003 2003 2007# Reporting % Target # Reporting % TargetEO 6.4 Post-marketing surveillance of medicine safety ma<strong>int</strong>ained and strengthened through the ongoing development of pharmacovigilancecentres and their involvement in <strong>int</strong>ernational adverse drug reaction monitoring systemsCountries monitoring adverse drug reactions 56/191 29% 35% 72/192 38% 45%EO 6.5 Use of substandard and counterfeit <strong>medicines</strong> reduced as a result of the development and application of effective strategies to detect theexistence and combat the production and circulation of such productsCountries with >10% of tested <strong>medicines</strong> failing quality tests na na na 20/71 28% 25%EO 6.6 Prequalification (initial assessment, ongoing monitoring and prequalification) of products and manufacturers of <strong>medicines</strong> for prioritydiseases; and of quality control laboratories, as appropriate, through procedures and guidelines appropriate for this activityNumber of products assessed and approved na na na 93 na naEO 6.7 Safety of new priority and neglected <strong>medicines</strong> enhanced through training workshops and increased capacity to assess safety issuesCountries participating in training programmes for <strong>int</strong>roducingnew therapies for priority and neglected diseases, e.g. malaria andAIDS0 na na 7 na 20EO 6.8 Regulatory harmonization monitored and promoted as appropriate, and networking initiatives developed, to facilitate and improveregulatory processes in countriesNumber of countries participating in harmonization initiativessupported financially and technically by WHOna na na 15/191 8% 18%EO 7.1 Rational use of <strong>medicines</strong> by health professionals and consumers advocatedCountries where the promotion of the rational use of <strong>medicines</strong> iscoordinated at the national government levelna na na 93/127 73% 75%EO 7.2 Essential <strong>medicines</strong> list, clinical guidelines and formulary process developed and promotedCountries with national list of essential <strong>medicines</strong> updated with<strong>int</strong>he last 5 years129/175 74% 75% 82/114 72% 75%Countries with treatment guidelines updated within the last 5 years 60/90 67% 70% 47/76 62% 65%EO 7.3 Independent and reliable <strong>medicines</strong> information identified, disseminated and promotedCountries with a national <strong>medicines</strong> information centre able toprovide independent information on <strong>medicines</strong> to prescribersand/or dispensersCountries with a <strong>medicines</strong> information centre/service accessibleto consumers62/123 50% 59% 53/129 41% 50%na na na 45/127 35% 40%EO 7.4 Responsible ethical <strong>medicines</strong> promotion for health professionals and consumers encouragedCountries with basic system for regulating pharmaceuticalpromotion92/132 70% 80% 83/113 73% 76%EO 7.5 Consumer education enhanced in recognition of the growing significance of self-medication and of consumer access to knowledge andadvice of variable qualityCountries that have implemented a national consumer educationcampaign in the last two yearsna na na 72/120 60% 60%EO 7.6 Drug and therapeutics committees promoted at institutional and district/national levelsCountries with DTCs in the majority of regions/provinces na na na 32/96 33% 40%EO 7.7 Training in good prescribing and dispensing practices promotedCountries that include the concept of essential <strong>medicines</strong> in basiccurricula for medicine and/or pharmacyna na na 72/88 82% 85%EO 7.8 Practical approaches to contain antimicrobial resistance developed based on the WHO Global Strategy to Contain AntimicrobialResistanceCountries with national <strong>strategy</strong> to contain antimicrobial resistance na na na 37/113 33% 40%EO 7.9 Identification and promotion of cost-effective strategies to promote rational use of <strong>medicines</strong>Countries that have undertaken a national assessment/study of therational use of <strong>medicines</strong>* Data collected from Traditional Medicine Surveyna na na 57/97 59% 60%