A Tutorial on Thermodynamics - University of Illinois at Urbana ...
A Tutorial on Thermodynamics - University of Illinois at Urbana ...
A Tutorial on Thermodynamics - University of Illinois at Urbana ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
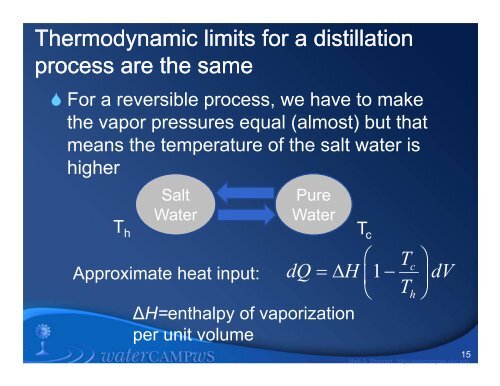
Thermodynamic limits for a distill<strong>at</strong>i<strong>on</strong>process are the same For a reversible process, we have to makethe vapor pressures equal (almost) but th<strong>at</strong>means the temper<strong>at</strong>ure <strong>of</strong> the salt w<strong>at</strong>er ishigherSaltW<strong>at</strong>erPureW<strong>at</strong>erT h T c TcApproxim<strong>at</strong>e he<strong>at</strong> input: 1∆H=enthalpy <strong>of</strong> vaporiz<strong>at</strong>i<strong>on</strong>per unit volumedQ H dV Th15Mark A. Shann<strong>on</strong> http://w<strong>at</strong>ercampws.uiuc.edu